Ferrous air oxidation reaction catalyst
A technology of air oxidation and catalyst, which is applied in the direction of physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, oxidized water/sewage treatment, etc., which can solve the problems of low cost and long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
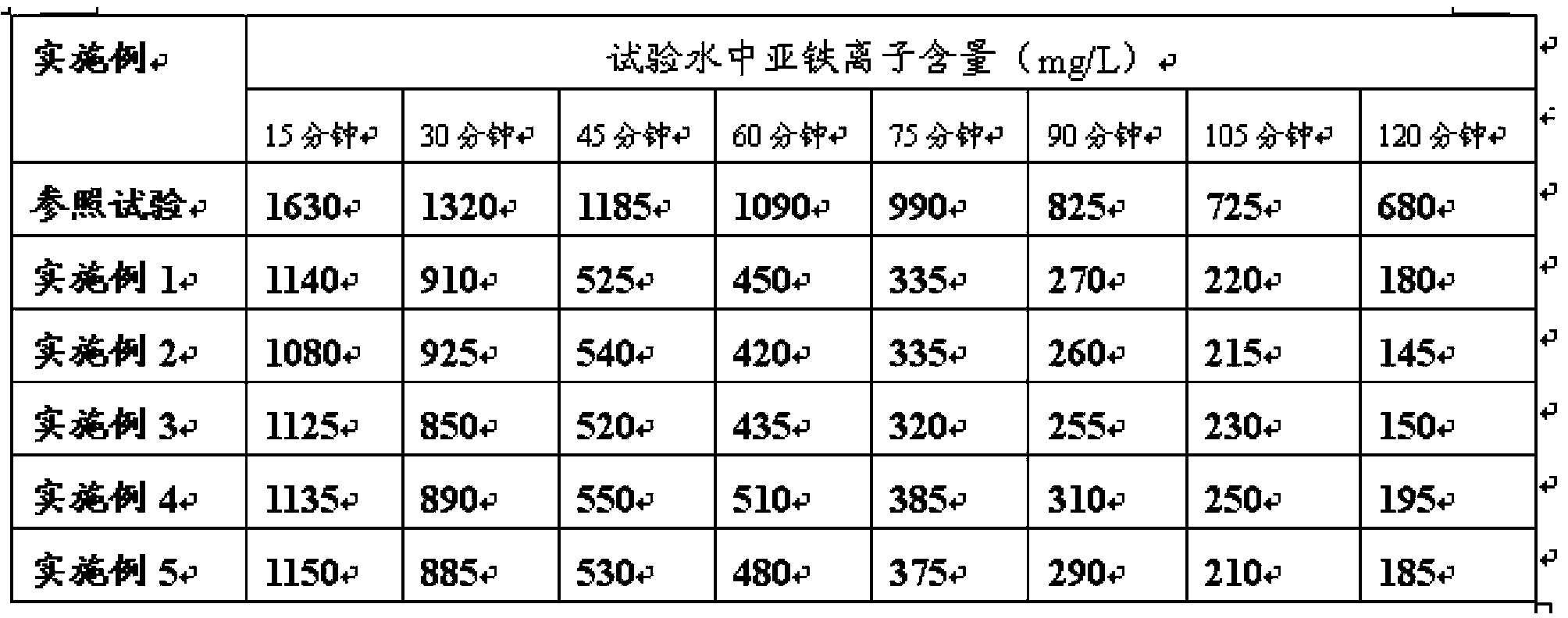
[0028] Preparation of ferrous oxidation reaction catalyst (by weight): 20 parts of sodium nitrite, 15 parts of copper sulfate, 5 parts of nitric acid, 15 parts of citric acid, 5 parts of hydroxyethylene diphosphonic acid, about 0.5 parts of anti-mildew agent, 39.5 parts of deionized water were added into 1000ml beakers, and the catalyst was obtained by stirring evenly.
[0029] Preparation of test water: Weigh 4.536 g of ferrous chloride, dissolve it in 1000 ml of deionized water, adjust the water to pH 1.0 with hydrochloric acid, and obtain simulated pickling wastewater. The water is neutralized with milk of lime to a pH value of 5-6 to obtain test water to simulate on-site acid wastewater conditions.
[0030] Test process: use the air oxidation method to oxidize ferrous iron, add 200ml of ferrous oxidation reaction catalyst to the test water, fully aerate and stir the beaker, and take samples every 15 minutes to analyze the content of ferrous ions.
Embodiment 2
[0032] Preparation of ferrous oxidation reaction catalyst (by weight): 25 parts of potassium nitrite, 15 parts of copper chloride, 3 parts of nitric acid, 15 parts of citric acid, 5 parts of hydroxyethylene diphosphonic acid, about 0.5 parts of anti- Mold agent, 36.5 parts of deionized water were added into 1000ml beakers, and the catalyst was obtained by stirring evenly.
[0033] Test water and test process refer to embodiment 1.
Embodiment 3
[0035] Preparation of ferrous oxidation reaction catalyst (by weight): 25 parts of sodium nitrite, 15 parts of copper nitrate, 4 parts of nitric acid, 15 parts of tartaric acid, 4 parts of diethylenetriaminepentamethylenephosphonic acid, about 0.5 parts of Antifungal agent, 36.5 parts of deionized water were added into a 1000ml beaker, and stirred evenly to obtain the required catalyst.
[0036] Test water and test process refer to embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com