Preparation method of levetiracetam
A technology of ethyl and ethanol, applied in the direction of organic chemistry, can solve the problems of 4-chlorobutyryl chloride instability, strong base racemization, easy hydrolysis, etc., and achieve stable yield, small amount of solvent, and low solvent toxicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
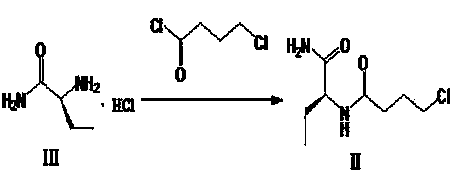
[0027] Step 1): Preparation of (S)-N-[1(aminocarbonyl)propyl]-4-chlorobutanamide (II)
[0028] Add (s)-2-aminobutyramide hydrochloride 2.5Kg (18.04mol), potassium carbonate 6220g (45.10mol), ethanol 30L into a 50L reactor and stir overnight, then add 4-chlorobutyryl chloride 3050g (21.65mol) dropwise , Stir for 30 minutes after dropping, TLC detection (developer: ethyl acetate / acetone=3 / 1, RfIII=0.1, RfII=0.7), filter after reaction, concentrate the filtrate to dryness under reduced pressure at 45°C, add acetic acid to the residue Ethyl ester / petroleum ether (1 / 4) 20L was beaten for 1-2 hours, filtered, and the solid was air-dried at 35°C overnight to obtain 2920g of white solid (II), with a yield of >74.2%.
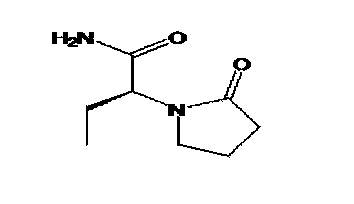
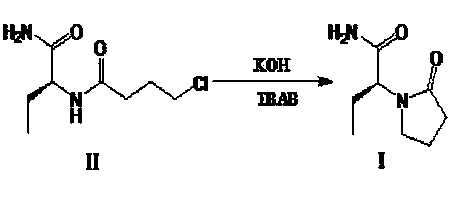
[0029] Step 2: Preparation of (S)-α-ethyl-2-oxo-1-pyrrolidineacetamide (levetiracetam) (I)
[0030] Add (S)-N-[1(aminocarbonyl)propyl]-4-chlorobutanamide (II) 5760g (27.9mol), dichloromethane 45L, tetrabutylammonium bromide 463g (1.4 mol), stir and mix, lower the tem...
Embodiment 2
[0034] The key intermediate is (S)-N-[1(aminocarbonyl)propyl]-4-chlorobutanamide (II) quality control method:
[0035] The TLC method detected basically a single spot, and the HPLC detection purity was greater than 95%.
[0036] TLC detection conditions: developer: ethyl acetate / acetone; volume ratio = 1:3; color development method: phosphomolybdic acid spraying, high temperature color development.
[0037] HPLC detection conditions:
[0038] Instrument: Shimadzu 20A series high performance liquid chromatography
[0039] Chromatographic column: Ymc-pack ODS-AQ column (4.6′150mm, 3μm)
[0040] Buffer salt: 0.26g potassium dihydrogen phosphate, add water to 1000ml, adjust pH to 5.50 with 2% potassium hydroxide
[0041] Mobile phase A: buffer salt-acetonitrile=19:1
[0042] Mobile Phase B: Acetonitrile
[0043] Flow rate: 0.9ml / min;
[0044] Injection volume: 10μl
[0045] Column temperature: room temperature
[0046] Detection wavelength: 205nm
[0047] Elution gradient...
Embodiment 3
[0050] Step 1): Preparation of (S)-N-[1(aminocarbonyl)propyl]-4-chlorobutanamide (II)
[0051] Add (s)-2-aminobutyramide hydrochloride 2.5Kg (18.04mol), potassium carbonate 6220g (45.10mol), ethanol 50L into a 50L reactor and stir overnight, then add 4-chlorobutyryl chloride 3050g (21.65mol) dropwise , Stir for 30 minutes after dropping, TLC detection (developer: ethyl acetate / acetone=3 / 1, RfIII=0.1, RfII=0.7), filter after reaction, concentrate the filtrate to dryness under reduced pressure at 45°C, add acetic acid to the residue Ethyl ester / petroleum ether (1 / 4) 20L was beaten for 1-2 hours, filtered, and the solid was air-dried at 35°C overnight to obtain 2920g of white solid (II), with a yield of >74.2%.
[0052] Step 2: Preparation of (S)-α-ethyl-2-oxo-1-pyrrolidineacetamide (levetiracetam) (I)
[0053]Add (S)-N-[1(aminocarbonyl)propyl]-4-chlorobutanamide (II) 5760g (27.9mol), dichloromethane 45L, tetrabutylammonium bromide 463g (1.4 mol), stir and mix, lower the te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com