Method for catalyzing olefin epoxidation by molybdenum polyoxometallate
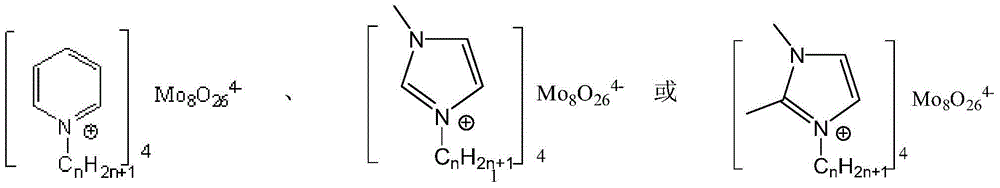
A technology for oxo-acid salts to catalyze olefins and polyoxometalates, which can be used in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc. Use and other problems to achieve the effect of solving the problem of recycling and reuse, ensuring the catalytic effect, and reducing the cost of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
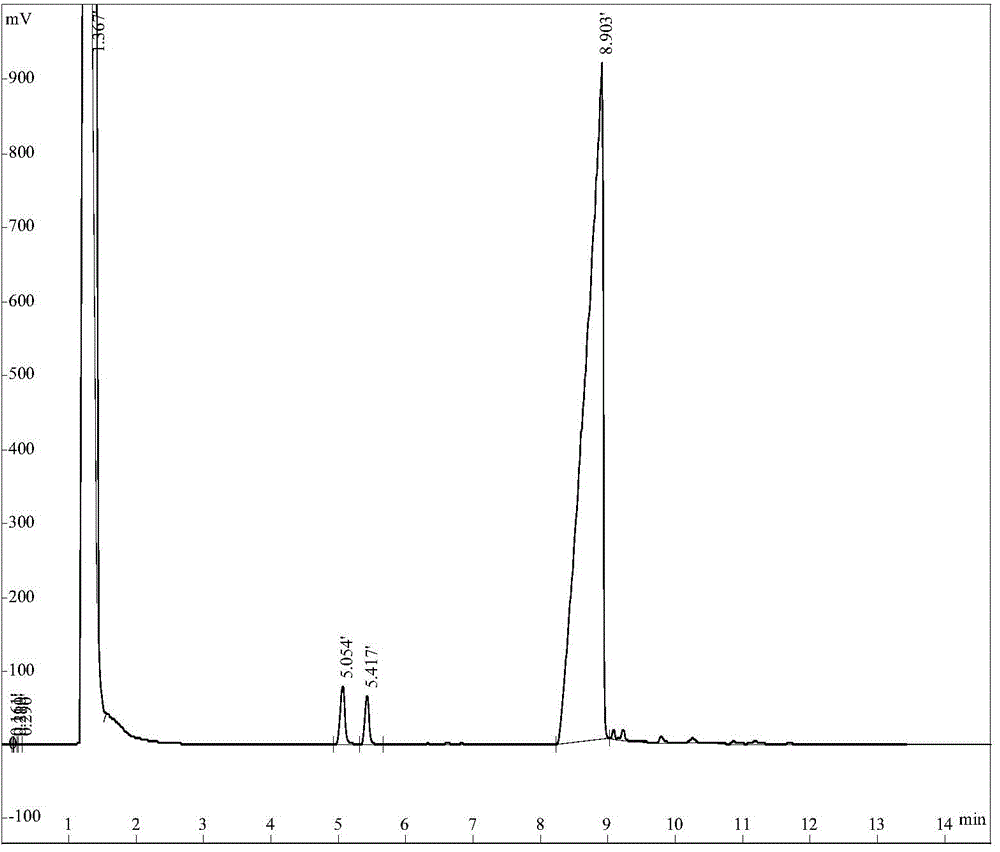
Image
Examples
Embodiment 1
[0019] Embodiment 1: Cyclooctene epoxidation method catalyzed by molybdenum polyoxometalate
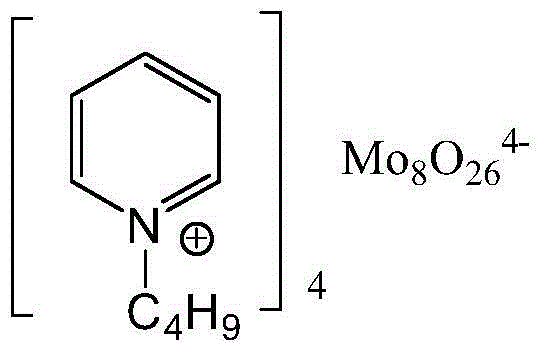
[0020] (1) Catalyst 1-butylpyridine molybdate ([C 4 py] 4 Mo 8 o 26 ) preparation
[0021] The structure of 1-butylpyridine molybdate is shown in the following formula:
[0022]
[0023] Its synthesis method is as follows:
[0024] (1) Add a certain amount of pyridine and n-butane bromide in a molar ratio of about 1:1.2 into a round-bottomed flask at room temperature. 2 Stir and react at 40°C for 1 hour in an oil bath under protection, then heat to 70°C and reflux for 12 hours to obtain the reaction intermediate product [C 4 py]Br, adding a mixture of acetonitrile and ethyl acetate (volume ratio: 1:2) for recrystallization, and then washing with n-hexane for 2 to 3 times, then distilling and drying with a rotary evaporator to obtain pure [C 4 py] Br intermediate;
[0025] (2) A certain amount of diluted [C 4 py]Br aqueous solution was exchanged through a cation exchange re...
Embodiment 2
[0032] Embodiment 2: Cyclooctene epoxidation method catalyzed by molybdenum polyoxometalate
[0033] (1) Catalyst 1-hexyl-3-methylimidazolium molybdate ([C 6 mim] 4 Mo 8 o 26 ) preparation
[0034] The structure of 1-hexyl-3-methylimidazolium molybdate is shown in the following formula:
[0035]
[0036] Its synthesis method is as follows:
[0037] (1) Add a certain amount of N-methylimidazole and n-hexane bromide in a molar ratio of about 1:1.2 into a round-bottomed flask at room temperature. 2 Stir and react at 40°C for 1 hour in an oil bath under protection, then heat to 70°C and reflux for 12 hours to obtain the reaction intermediate product [C 6 mim]Br, to which a mixture of acetonitrile and ethyl acetate (1:2 by volume) was added for recrystallization, the obtained crystals were washed 2 to 3 times with n-hexane, and dried in vacuum to obtain pure [C 6 mim]Br intermediate;
[0038] (2) A certain amount of diluted [C 6mim] Br aqueous solution is exchanged by a...
Embodiment 3
[0044] Example 3: Cyclooctene epoxidation catalyzed by molybdenum polyoxometalates
[0045] (1) Catalyst 1,2-dimethyl-3-ethylimidazolium molybdate ([C 2 dmim] 4 Mo 8 o 26 ) preparation
[0046]
[0047] Its preparation method is as follows:
[0048] (1) Add a certain amount of 1,2-dimethylimidazole to ethyl bromide in a molar ratio of about 1:1.2 into a round-bottomed flask at room temperature. 2 Stir and react at 40°C for 1 hour in an oil bath under protection, then heat to 70°C and reflux for 12 hours to obtain the reaction intermediate product [C 2 dmim]Br, recrystallized from a mixture of acetonitrile and ethyl acetate (volume ratio 1:2), washed 2 to 3 times with an appropriate amount of n-hexane, and distilled and dried by a rotary evaporator to obtain pure [C 2 dmim]Br;
[0049] (2) A certain amount of diluted [C 2 dmim]Br aqueous solution was exchanged through a cation exchange resin column, and the effluent solution was collected in batches and treated with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com