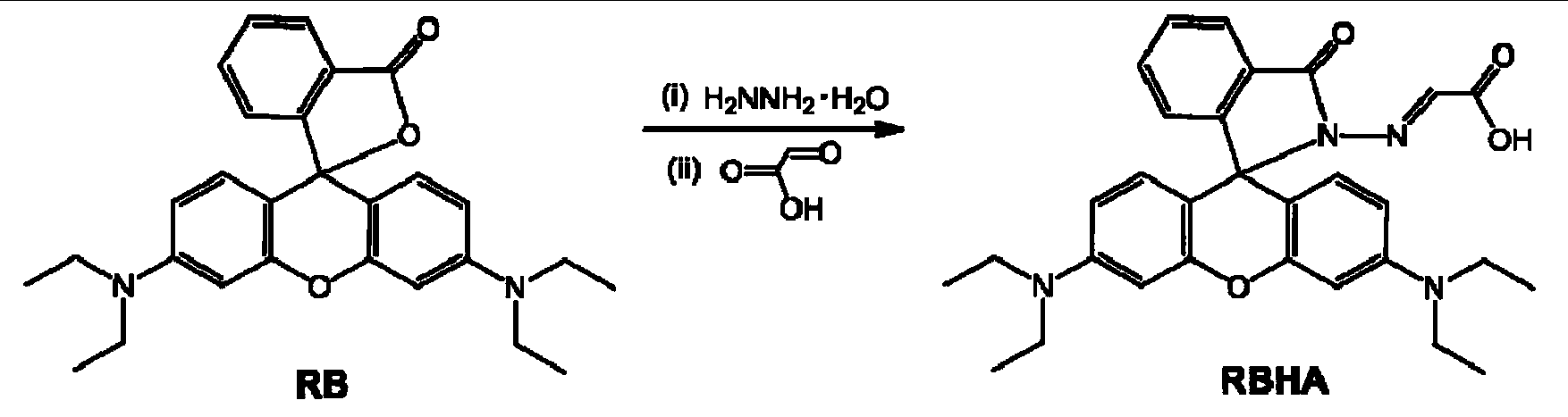Reactive rhodamine fluorescent probe for detecting mercury ions, and preparation method thereof
A fluorescent probe and reaction-type technology, applied in the field of fluorescent probe materials and their preparation, can solve the problem of less mercury ion fluorescent probes, and achieve the effects of good water solubility, simple preparation method and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Synthesis of rhodamine B hydrazide: Rhodamine B (5.0g) and hydrazine hydrate (5.0ml) were refluxed in 30ml ethanol for 2 hours, the solvent was removed by rotary evaporation, water was added and 1M dilute hydrochloric acid was used to adjust to acidic (pH =2), dissolve all the solid residues, and then use 1M sodium hydroxide solution to adjust to weak alkalinity (pH=9-10); filter the precipitated precipitate, wash with water, purify, and dry in vacuo to obtain 4.0g rhodamine B yl Hydrazine, yield 78%. ESI-MS(M+H) + :m / z calcd457.3;found457.4. (2) Synthesis of reactive fluorescent probe RBHA: rhodamine B hydrazide (1.0 g) and glyoxylic acid (0.4 g) were dissolved in 15 mL of methanol, and stirred for 10 hours at room temperature under nitrogen protection. The precipitate was filtered and washed with methanol three times, and dried in vacuum to obtain 0.85 g of the target product RBHA with a yield of 76%.
[0028] 1 H NMR (400MHz, DMSO-d 6 ),d(ppm):1.08(t,J=8.0Hz...
Embodiment 2
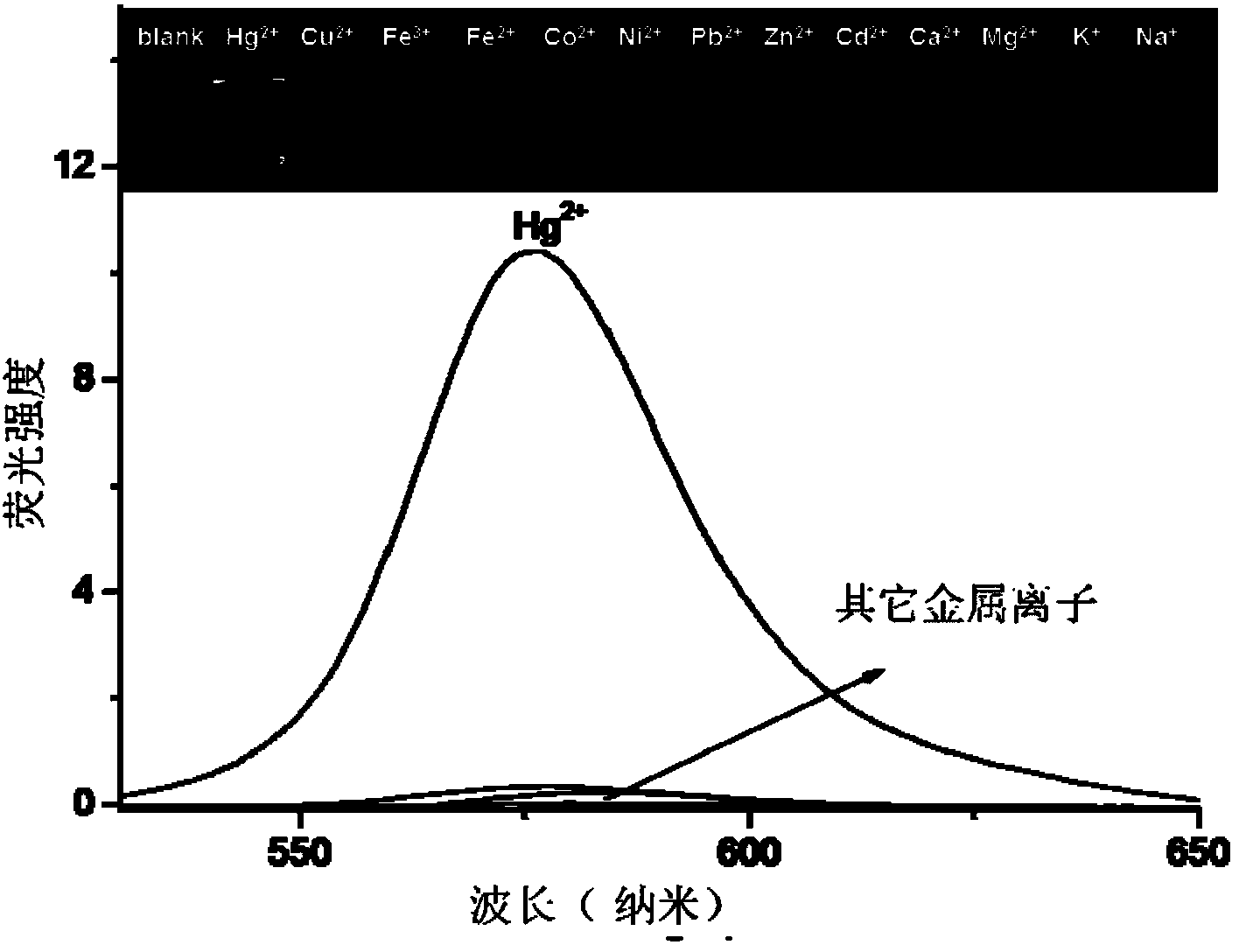
[0031] The selectivity of the fluorescent probe RBHA for the fluorescence detection of mercury ions:
[0032] Tris-HCl buffer solution with pH=7.4 was used to control the experimental conditions.
[0033] Add 0.1ml ethanol solution (1mM) of fluorescent probe RBAH and 2ml aqueous solution of metal ions to be tested (both metal ion concentration is 1mM) to different 10ml colorimetric tubes respectively, dilute to 10ml with Tris-HCl buffer solution, place 1 hour. Transfer 3ml of the working solution to a 1cm fluorescence cuvette to measure the fluorescence spectrum, the excitation wavelength is 520nm. In the blank experiment, no metal ions were added to the above solution. Selective detection of mercury ions such as figure 2 shown (inset is a photograph of fluorescence emission). Visible fluorescence is significantly enhanced only in the presence of mercury ions, indicating the high selectivity of the fluorescent probe RBAH involved in the present invention to mercury ions. ...
Embodiment 3
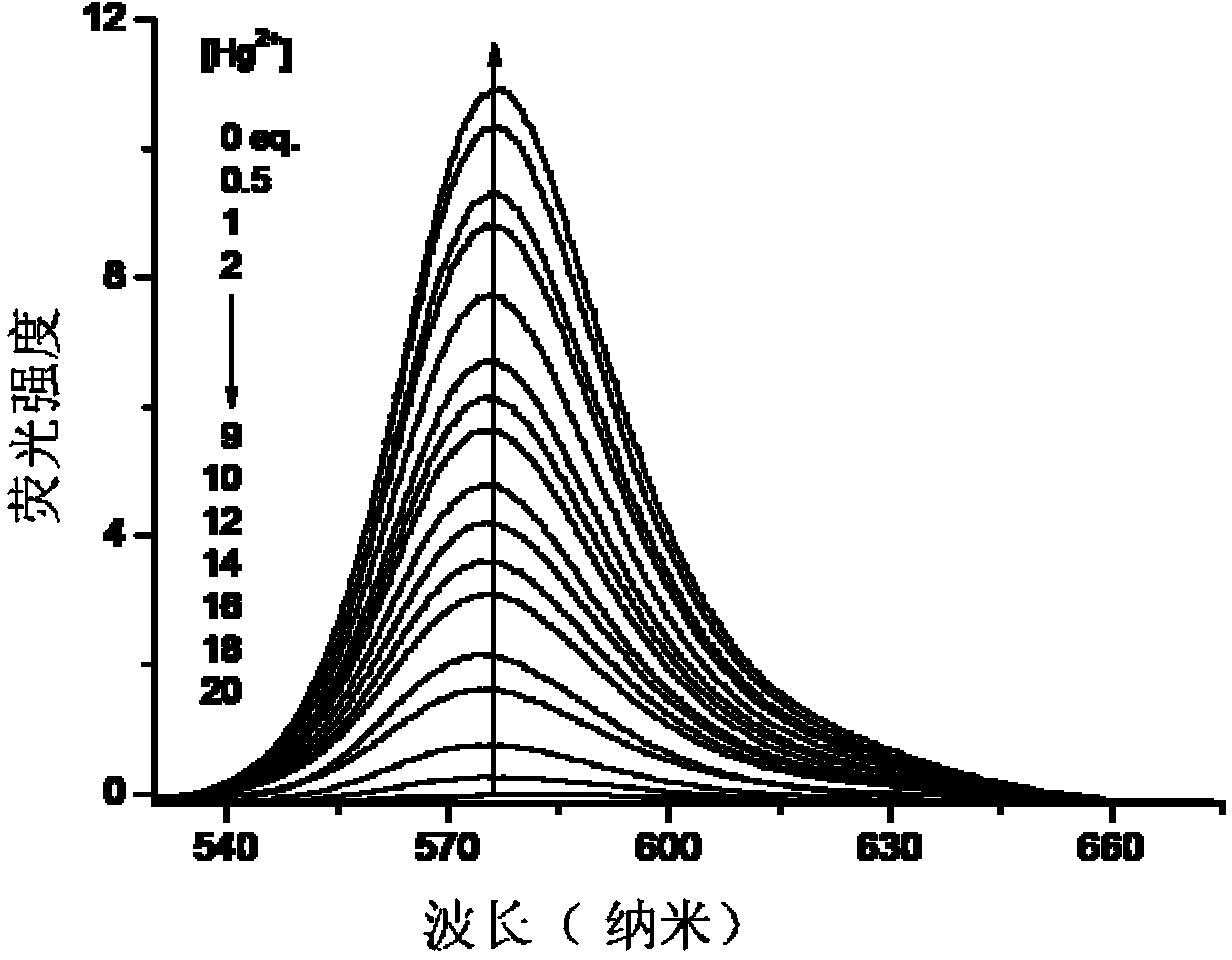
[0035] Quantitative fluorescent detection of mercury ions by fluorescent probe RBAH:
[0036]Add 0.1ml ethanol solution (1mM) of fluorescent probe RBAH and 0-3.0ml different volumes of 1mM mercury ion aqueous solution to different 10ml colorimetric tubes respectively, and dilute to 10ml with Tris-HCl buffer solution, and place for 1h. After constant volume, the concentration of the fluorescent probe is 10 mM, and the concentration of mercury ions is 0-300 mM. Transfer 3ml of the working solution to a 1cm fluorescence cuvette to record the fluorescence spectrum and read and record the fluorescence intensity at 576nm. The fluorescence intensity and the corresponding mercury ion concentration data were input into the software Origin8 for fitting, and a linear working curve was obtained in the range of mercury ion concentration 5-200mM, and the linear regression constant was 0.9825, indicating that the probe can quantitatively detect the concentration of mercury ions. The change ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com