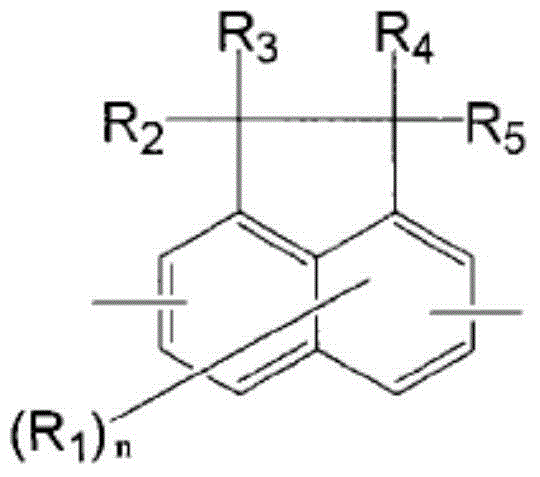
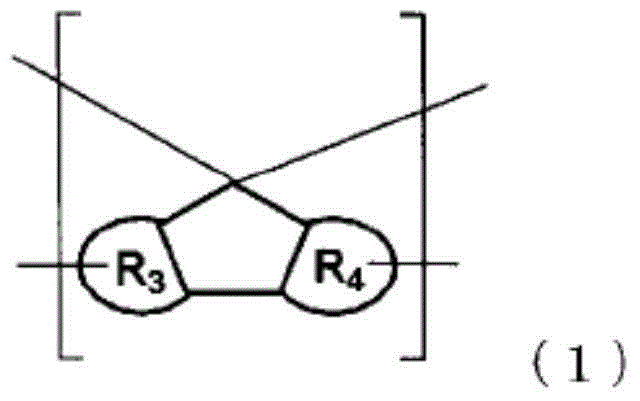
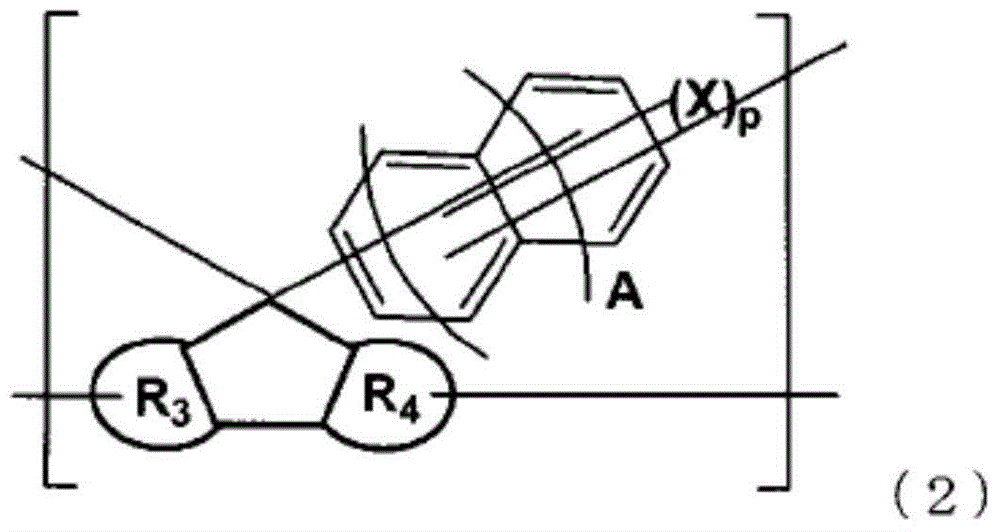
Resin having fluorene structure and underlayer film-forming material for lithography
A technology of resin and benzofluorene, which is applied in the field of the lower layer film for lithography, and achieves the effects of high heat resistance, high solvent solubility, and high carbon concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0220] Hereinafter, the present invention will be described in more detail based on synthesis examples and examples, but the present invention is not limited to these examples.
[0221] · Carbon concentration and oxygen concentration in the resin
[0222] The carbon concentration and oxygen concentration (mass %) in the resin were measured by organic elemental analysis.
[0223] Device: CHN CORDER MT-6 (manufactured by Yanaco Analytical Industry Co., Ltd.)
[0224] ·Molecular weight
[0225] The polystyrene-equivalent weight average molecular weight (Mw) and number average molecular weight (Mn) were determined by gel permeation chromatography (GPC) analysis, and the degree of dispersion (Mw / Mn) was determined.
[0226] Device: Shodex GPC-101 (manufactured by Showa Denko Co., Ltd.)
[0227] Chromatographic column: KF-80M×3
[0228] Eluent: THF1ml / min
[0229] Temperature: 40°C
[0230] ·Structural analysis
[0231] Structural analysis was carried out in combination with ...
Synthetic example 1
[0259] Into a 200-ml three-necked flask with an internal volume equipped with a serpentine condenser, a thermometer, and a stirring blade, 33 g (0.2 mol) of fluorene (manufactured by Acros Organics) was added under nitrogen flow, and the temperature was raised to 230° C. for 12 hours. 2 ml of methanesulfonic acid (manufactured by Kanto Chemical Co., Ltd.) was added to the reaction liquid at intervals of 1 hour from the beginning of the reaction, and a total of 8 additions were made. Then, 80 g of methyl isobutyl ketone (manufactured by Kanto Chemical Co., Ltd.) and 40 g of anisole (manufactured by Kanto Chemical Co., Ltd.) were added to the reaction solution to dilute, neutralized and washed with water, and the solvent was removed under reduced pressure, thereby 13 g of resin (NF-1) of synthesis example 1 which is a target object were obtained.
[0260] As a result of GPC analysis, Mn: 830, Mw: 3040, Mw / Mn: 3.66. As a result of organic element analysis, the carbon concentrati...
Synthetic example 2
[0263] Into a four-neck flask with an internal volume of 1 L equipped with a serpentine condenser, a thermometer, and a stirring blade, 128 g (1.0 mol) of naphthalene (manufactured by Kanto Chemical Co., Ltd.) and 9-fluorenone (manufactured by Acros Organics) were added under a nitrogen stream. 180 g (1.0 mol), heated up to 230° C. and reacted for 8 hours. 2.5 ml of methanesulfonic acid (manufactured by Kanto Chemical Co., Ltd.) was added to the reaction solution at intervals of 1 hour from the beginning of the reaction, and a total of 8 additions were made. Then, 400 g of methyl isobutyl ketone (manufactured by Kanto Chemical Co., Ltd.) and 200 g of anisole (manufactured by Kanto Chemical Co., Ltd.) were added to the reaction solution to dilute, neutralized and washed with water, and the solvent was removed under reduced pressure, thereby 200 g of resin (NF-2) of the synthesis example 2 which is a target object was obtained.
[0264] As a result of GPC analysis, Mn: 625, Mw:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com