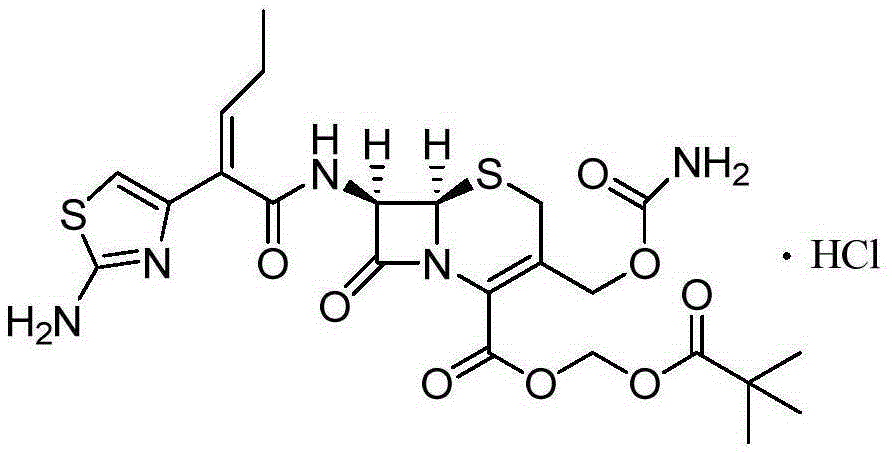
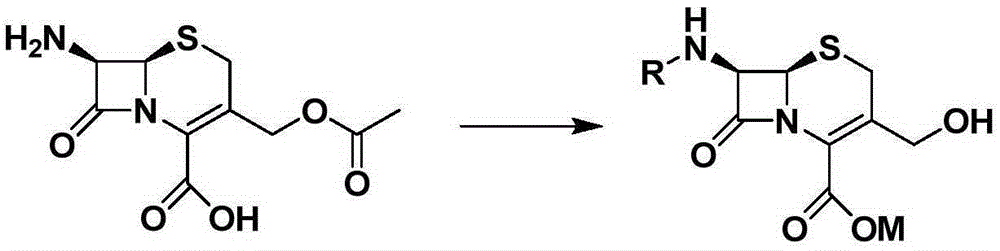
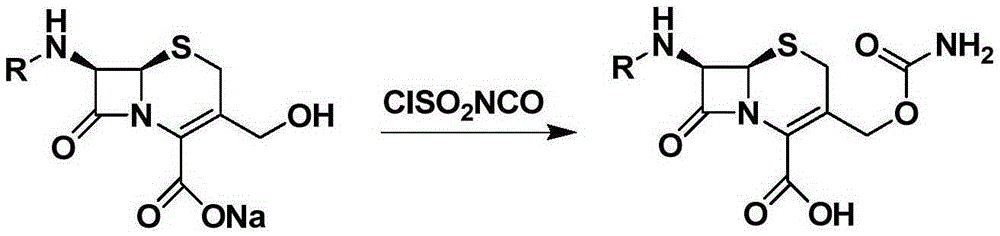
A kind of synthetic method of cefcapene hydrochloride
A technology of cefcapene pivoxil hydrochloride and cefcapene pivoxil hydrochloride, which is applied in the field of synthesis of cefcapene pivoxil hydrochloride, can solve the problems of low total yield, complexity, unfavorable large-scale production, etc., and achieve low cost, simple operation, and finished products The effect of high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Synthesis of 7-DACA and Boc-7-DACA sodium salt
[0036] Suspend 10.0 g of 7-ACA in 30 mL of methanol / water mixed solvent, cool to -10°C to 0°C, and then add 30 mL of 10% NaOH solution dropwise (by adding in batches). Keep the temperature stable, stir for 2h, follow the reaction by TLC, the raw material point 7-ACA disappears, that is, the reaction is over. Then add cold acetone solution to the reaction system, stir vigorously, gradually a light yellow solid precipitates out. Filtered, drained, and recrystallized with absolute ethanol to obtain the product 7-DACA with a yield of 86%.
[0037] 1 HNMR (D 2 O,400MHz):δ3.22-3.27(d,1H),3.43-3.47(d,1H).4.06(s,2H),4.85-4.86(dd,2H).
[0038] If the reaction is complete, add directly without processing (Boc) 2 O, the reaction was followed by TLC. If the reaction is not complete, alkali can be added appropriately. After the reaction was completed, EA was added to extract the organic phase three times, and cold...
Embodiment 2
[0040] Embodiment 2: o-chlorobenzoyl chloride protects the amino group
[0041] Suspend 10.0 g of 7-ACA in 30 mL of methanol / water mixed solvent, cool to -10°C to 0°C, and then add 30 mL of 10% NaOH solution dropwise (by adding in batches). Keep the temperature stable, stir for 2h, follow the reaction by TLC, the raw material point 7-ACA disappears, that is, the reaction is over. Then add cold acetone solution to the reaction system, stir vigorously, gradually a light yellow solid precipitates, filter and drain. Dissolve this solid in a mixed solvent of 1,4-dioxane / water (1:1), add a saturated solution of sodium bicarbonate to make the system alkaline, then add o-chlorobenzoyl chloride dropwise, and continue stirring. Saturated sodium bicarbonate solution was added, and the reaction was tracked by TLC. After the reaction was completed, a large amount of solid was precipitated by adding acetone, and washed with cold absolute ethanol to obtain the target product with a yield o...
Embodiment 3
[0042] Example 3: Amidation of Boc-7-DACA3-hydroxyl
[0043] in N 2Under protection, suspend Boc-7-DACA (5.0g) in DMF or acetonitrile solvent, lower the temperature from -20°C to -10°C, then slowly add the DMF solution of chlorosulfonyl isocyanate dropwise, keep the temperature, and stir for 1h. Slowly add 5 mL of cold purified water to quench the reaction, continue to stir for 3 h, extract with EA, wash with saturated sodium bicarbonate solution and saturated brine successively, dry, concentrate and recrystallize to obtain the target product with a yield of 68%. Dissolve this product in ethyl acetate, then pass through hydrochloric acid gas, and precipitate a solid while stirring, which is the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com