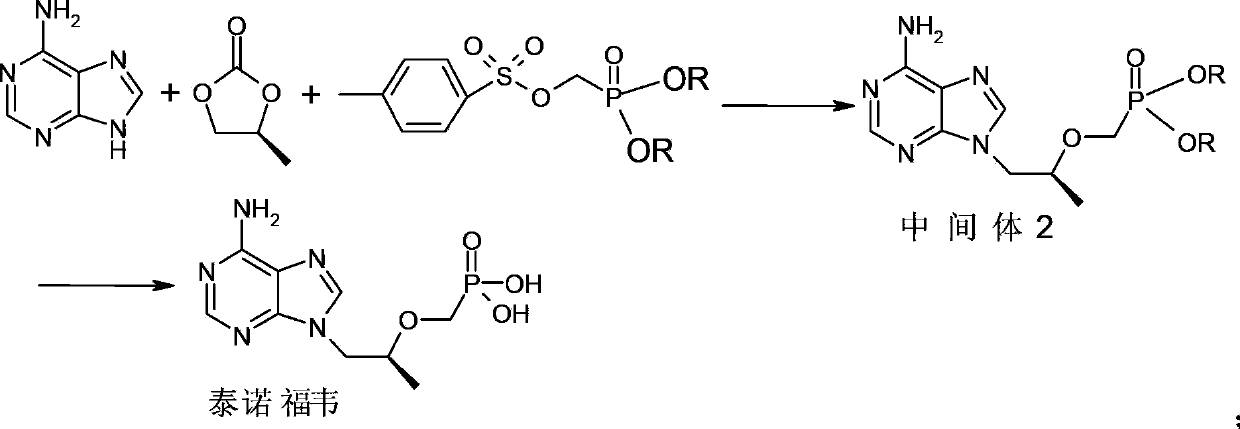
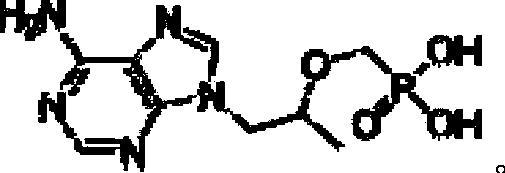
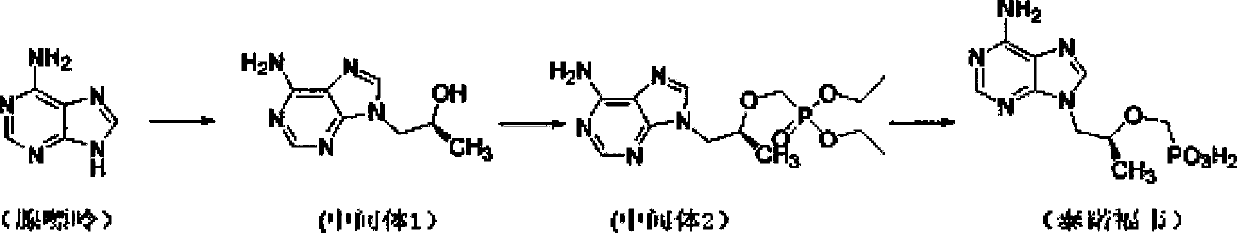
Process for preparing tenofovir
A process and adenine technology, applied in the field of drug synthesis, can solve problems such as unsuitability for industrial production, production cost environmental protection defects, harsh dissociation conditions, etc., and achieve the effects of simple operation, low cost, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
[0039] Dissolve adenine (17.4g, 0.13mol), potassium carbonate (21.5g, 0.15mol), R-propylene carbonate (15.8g, 0.15mol) in 50mL N,N-dimethylformamide, heat to 90~ 125°C, keep warm at 90-125°C for 3-6 hours; cool down to room temperature, add magnesium isopropoxide (15.7g, 0.14mol) under argon protection, heat up to 30-45°C, keep warm for 0.5-1 hour ; Add dimethyl p-toluenesulfonyloxymethylphosphonate (44.1g, 0.15mol) dropwise, and keep warm at 30-45°C to continue the reaction for 4-6 hours; the system is lowered to room temperature, and the reaction system is concentrated under reduced pressure to get viscous Intermediate 2 of the oily substance; add 124g of concentrated hydrochloric acid to the prepared intermediate 2, heat up to 90-110°C, and keep warm for 3-6 hours; cool down to room temperature, continue to react at room temperature for 1-2 hours, and filter with suction , the filter cake was washed twice with 150mL 5wt% dilute hydrochloric acid aqueous solution...
Embodiment 2
[0041]
[0042] Dissolve adenine (17.4g, 0.13mol), sodium carbonate (15.9g, 0.15mol), R-propylene carbonate (15.8g, 0.15mol) in 50mL of N-methylpyrrolidone, heat to 90-125°C, keep React at 90-125°C for 3-6 hours; cool down to room temperature, add magnesium tert-butoxide (23.8g, 0.14mol) under the protection of argon, heat up to 30-45°C, and keep warm for 0.5-1 hour; Diethyl toluenesulfonyloxymethylphosphonate (48.3g, 0.15mol), keep warm at 30-45°C and continue to react for 4-6 hours; lower the system to room temperature, concentrate the reaction system under reduced pressure to obtain a viscous oil in the middle Body 2; add 130g of concentrated sulfuric acid to the prepared intermediate 2, raise the temperature to 90-110°C, and keep it warm for 3-6 hours; cool down to room temperature, continue to react at room temperature for 1-2 hours, suction filter, and use the filter cake Wash twice with 150mL of 5wt% dilute sulfuric acid aqueous solution, adjust the pH of the filtrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com