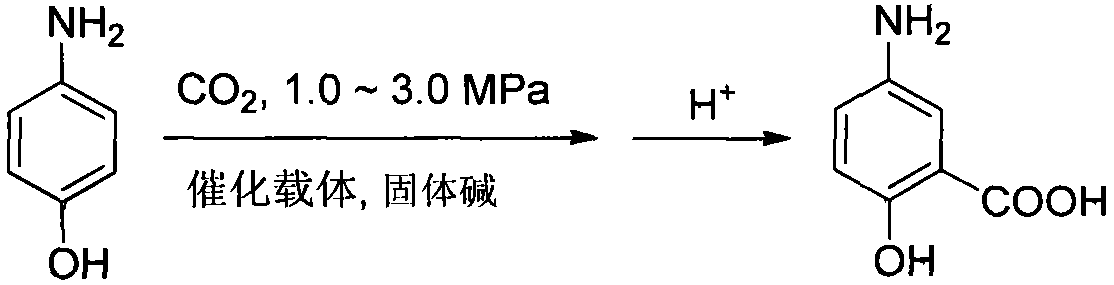
Preparation method of 2-hydroxy-5-aminobenzoic acid
A technology of aminobenzoic acid and aminobenzoate, applied in the preparation of organic compounds, chemical instruments and methods, cyanide reaction preparation, etc., can solve the problems of serious pollution of three wastes, difficult purification, unknown catalyst, etc., and achieve HPLC purity Good, easy to obtain raw materials, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: add 16g p-aminophenol, 43g NaCl, 47g Na in autoclave 2 CO 3 , After the feeding is completed, replace the reaction vessel with carbon dioxide gas three times, then press it to 1.0MPa, slowly raise the temperature to 200°C, and react at this temperature for 6 hours. After the reaction is finished, cool down to 50°C, release the pressure and open the reaction kettle, add 200mL sodium dithionite solution into the reaction kettle, stir for another 1-2 hours, filter out the insoluble matter, and adjust the pH of the filtrate to 1.0-6.0 with hydrochloric acid , a solid was precipitated, filtered to obtain a gray solid crude product. The crude product was slurried in water to obtain an off-white solid with a yield of 90% and a purity of 99.1%; melting point: 279-281° C. (dec).
[0026] 1 H NMR (400MHz, DMSO-d 6 ): δ=7.12(s, 1H, ArH), 6.86-6.83(m, 1H, ArH), 6.68(d, 1H, J=8.8Hz, ArH).
Embodiment 2
[0027] Embodiment 2: add 16g p-aminophenol, 11g KCl, 20g K in autoclave 2 CO 3 , After the feeding is completed, replace the reaction vessel with carbon dioxide gas three times, then press it to 3.0MPa, slowly raise the temperature to 180°C, and react at this temperature for 6 hours. After the reaction is finished, cool down to 50°C, release the pressure and open the reaction kettle, add 200mL sodium dithionite solution into the reaction kettle, stir for 1-2 hours, filter out the insoluble matter, and adjust the pH of the filtrate to 1.0-6.0 with acid , a solid was precipitated, filtered to obtain a gray solid crude product. The crude product was slurried in water to obtain an off-white solid with a yield of 88% and a purity of 99.3%.
Embodiment 3
[0028] Embodiment 3: add 16g p-aminophenol, 163g KCl, 162g K in autoclave 2 CO 3 , After the feeding is completed, replace the reaction vessel with carbon dioxide gas three times, then press it to 1.5MPa, slowly raise the temperature to 220°C, and react at this temperature for 6 hours. After the reaction is finished, cool down to 50°C, release the pressure and open the reaction kettle, add 200mL sodium dithionite solution into the reaction kettle, stir for 1-2 hours, filter out the insoluble matter, and adjust the pH of the filtrate to 1.0-6.0 with acid , a solid was precipitated, filtered to obtain a gray solid crude product. The crude product was slurried in water to obtain an off-white solid with a yield of 86% and a purity of 99.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com