Medicinal composition
一种组合物、药物的技术,应用在药物组合、纳米药物、杂环化合物有效成分等方向,能够解决未能得到等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
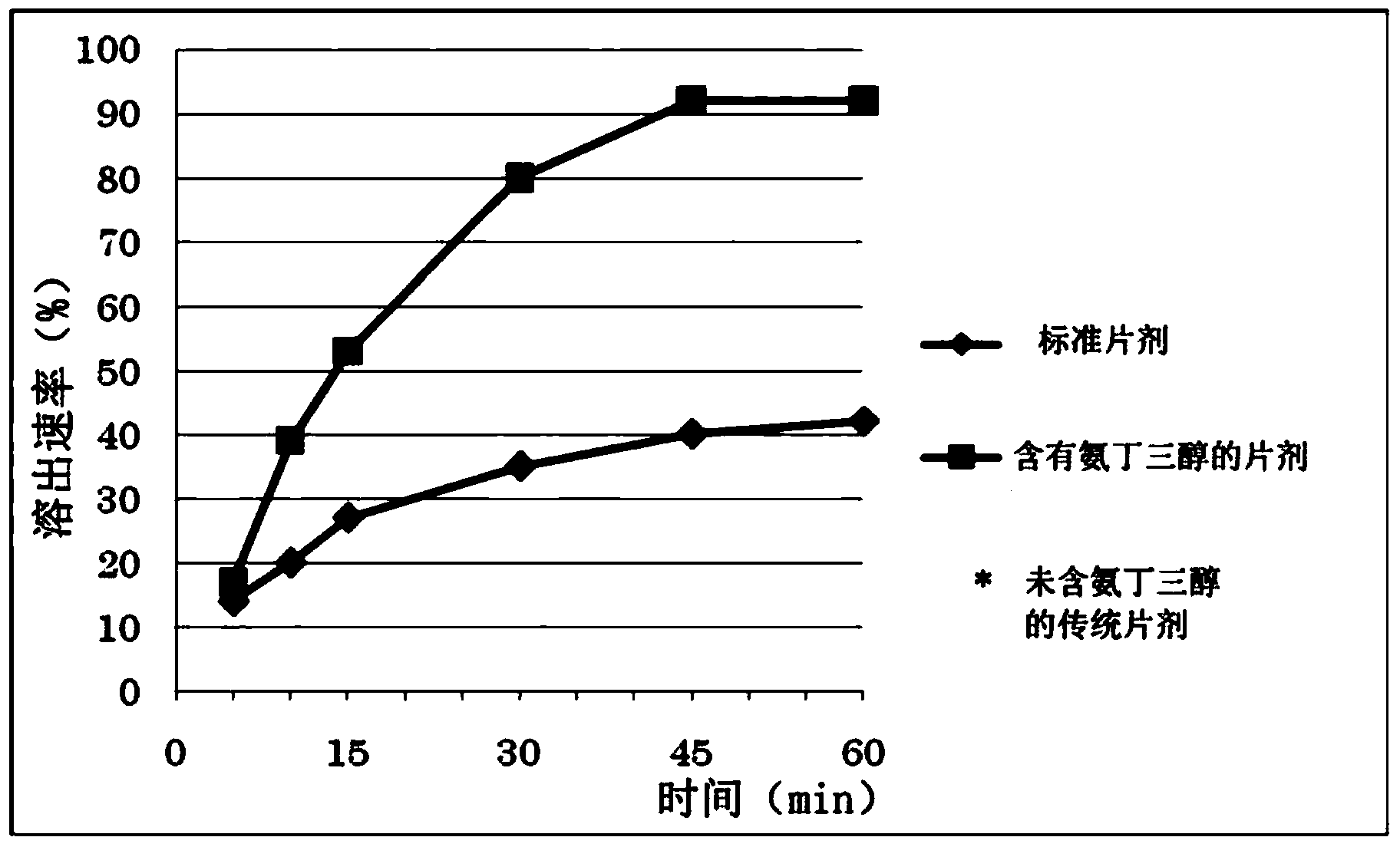
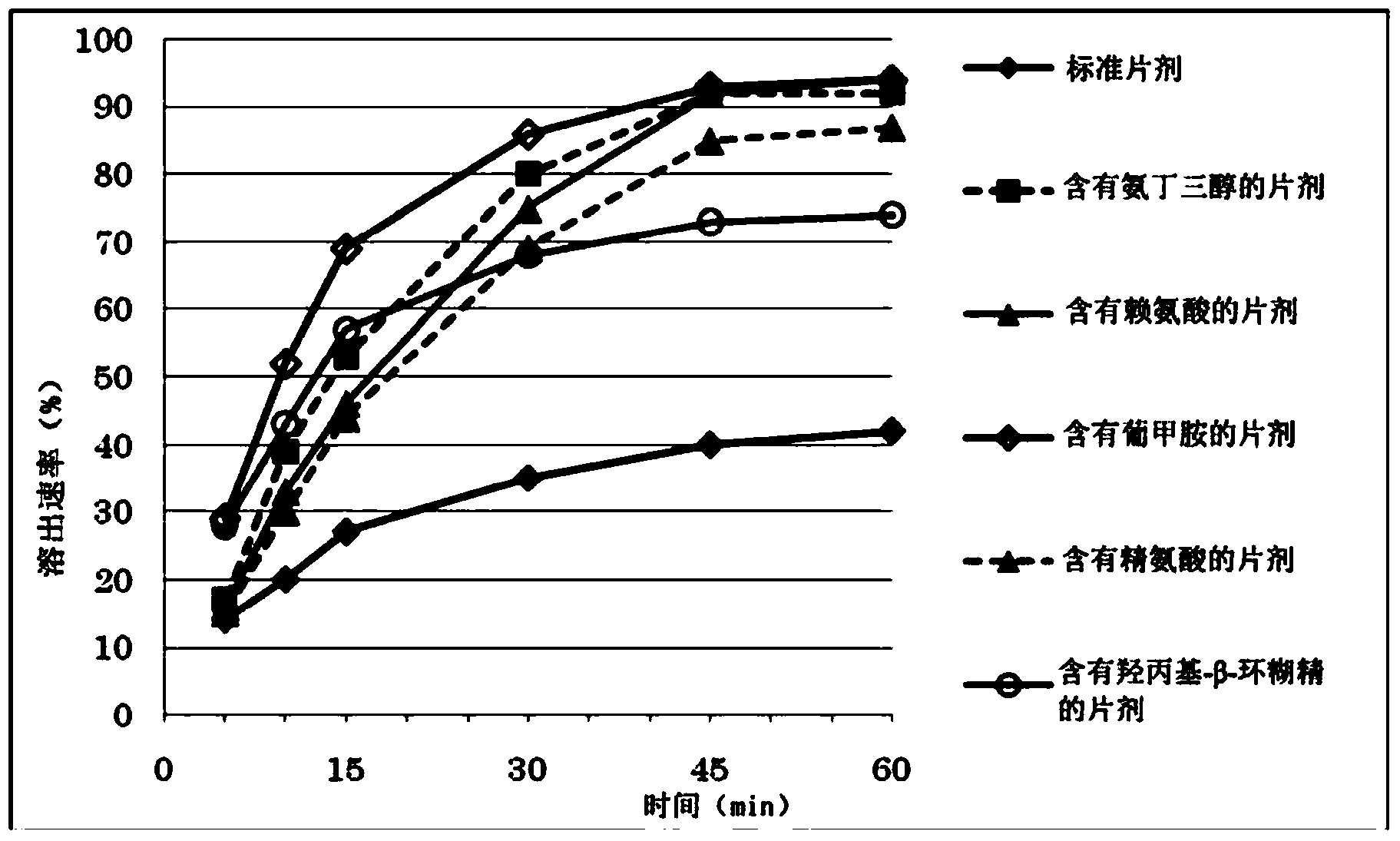
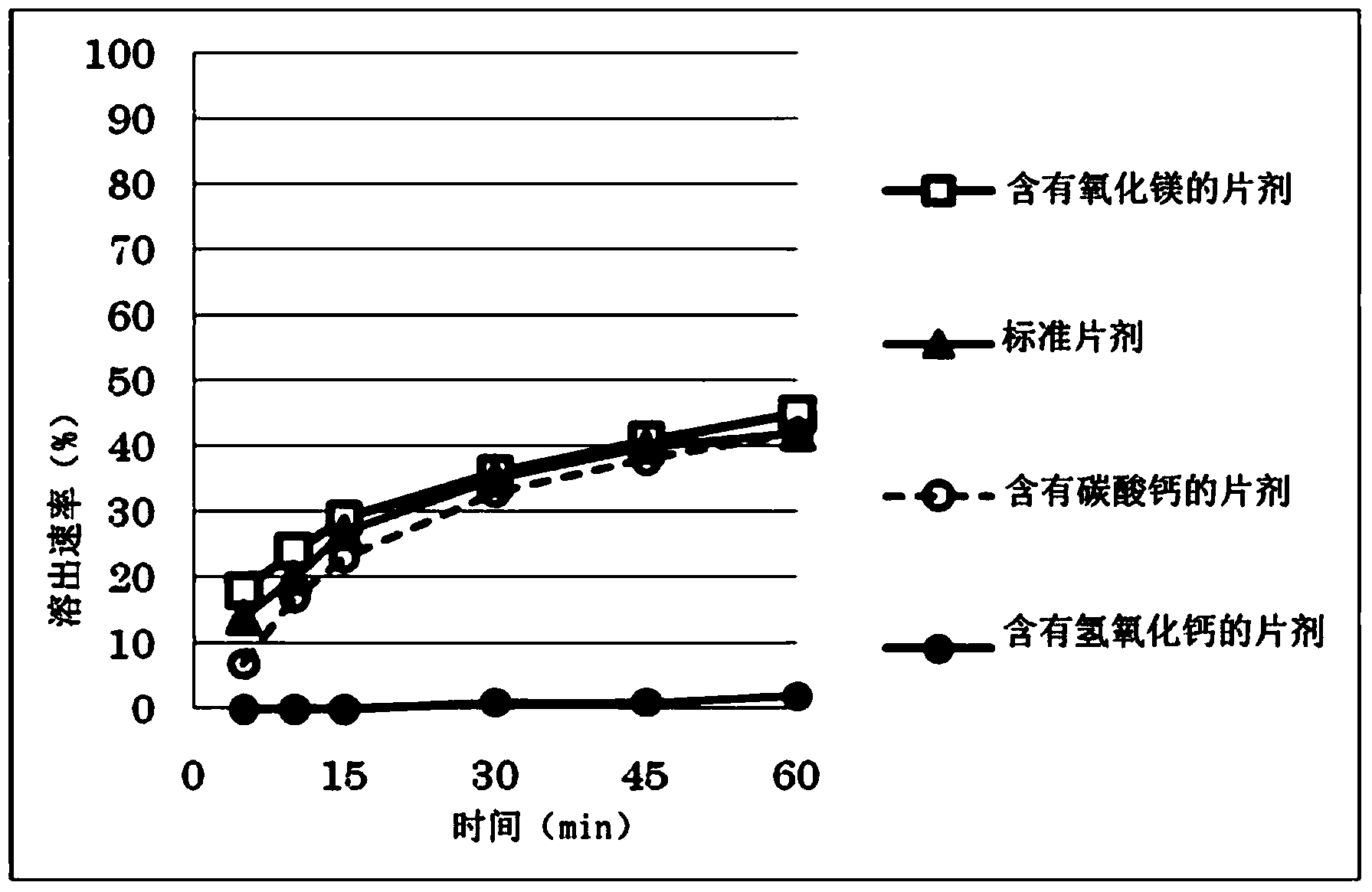
Image
Examples
Embodiment 1
[0161] [Example 1] Tablets of compound A containing trometamol as a basic amine
[0162] dosage form
[0163] The 60 mg tablet of Compound A containing tromethamine was prepared according to the dosage forms described in Table 1, Table 2 and Table 3. Tablets of other doses are prepared proportional to the dose according to the weight % of each ingredient. Tablets containing other basic amines such as triethanolamine, diethanolamine, monoethanolamine, etc. are prepared in a similar manner to the dosage form in tromethamine. In fact, tablets can be prepared by substituting other basic amines for tromethamine in Table 1, Table 2 and Table 3.
[0164] Manufacturing method 1
[0165] Each compound A that was ground through a 0.006-inch wheel sieve at 1800 rpm, tromethamine and mannitol both of which were ground through a 0.018-inch wheel sieve at 1800 rpm were weighed. Avicel PH102 was added to the PK-blender and blended for 1 minute to coat the surface. Compound A, milled tro...
Embodiment 2
[0181] [Example 2] Capsules of compound A containing trometamol as a basic amine
[0182] dosage form
[0183] 60 mg capsules of Compound A containing trometamol were prepared in the dosage form described in Table 4. Capsules of other doses are prepared in proportion to the dose according to the weight % of each ingredient. Capsules containing other basic amines such as triethanolamine, diethanolamine, monoethanolamine, etc. are prepared by a method similar to the dosage form in tromethamine. In fact, capsules were prepared by substituting other basic amines for trometamol in Table 4.
[0184] Manufacturing method
[0185] Mill Compound A, trometamol, lactose monohydrate, microcrystalline cellulose PH102, hydroxypropylmethylcellulose and cross- Sodium carboxymethyl cellulose for blending. A solution of polysorbate 80 dissolved in purified water was added to the granulator. The granular material was milled through a 0.25 inch wheel screen and dried on trays. The granular...
Embodiment 3
[0189] [Example 3] Tablets of compound A containing meglumine as a basic amine
[0190] dosage form
[0191] Tablets were prepared using meglumine instead of trometamol as described in Table 3.
[0192] Manufacturing method
[0193] Tablets were prepared by a method similar to Production Method 3 in Example 1. In fact, meglumine was used instead of trometamol as described in Table 3 to prepare 60 mg tablets of Compound A containing meglumine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com