Medicinal composition of recombinant carboxypeptidase G2
A technology of carboxypeptidase and composition, which is applied in the field of recombinant carboxypeptidase G2 pharmaceutical composition freeze-dried powder injection preparation and preparation thereof, can solve the problems of reduced purity, lack of availability, influence on efficacy and the like, and achieves low cost and increased Toxic side effects, easy to produce effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
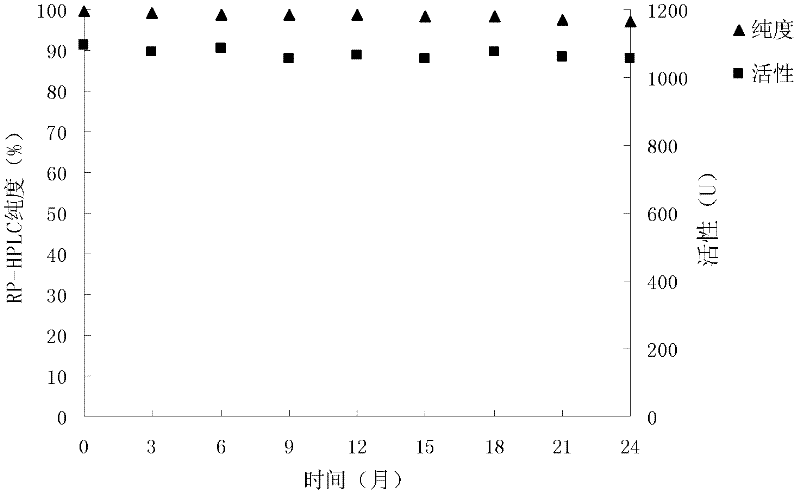
[0047] Embodiment 1: The impact of excipient concentration on preparation molding, solubility
[0048] Two excipients, mannitol and sorbitol, were investigated on the formability and reconstitution solubility of lyophilized products under different concentration conditions. The specific experimental process is: to contain 0.2mM ZnCl 2 25mM Tris-HCl buffer solution with a pH of 7.3 to prepare 20% mannitol and sorbitol; respectively get 5ml carboxypeptidase G2 stock solution (2000U / ml) and a certain amount of mannitol or sorbitol mother solution (0ml, 0.5ml . Enzyme G2 solution, divided into 1.1ml / cartridge, freeze-dried; at 25°C, after standing for 20 days, each sample was reconstituted with 1ml water for injection, and the solubility was observed, and analyzed by RP-HPLC:
[0049] 1. The influence of mannitol on the stability of preparations
[0050]
[0051]
[0052] 2. The effect of sorbitol on the stability of the preparation
[0053]
[0054] It can be seen fr...
Embodiment 2
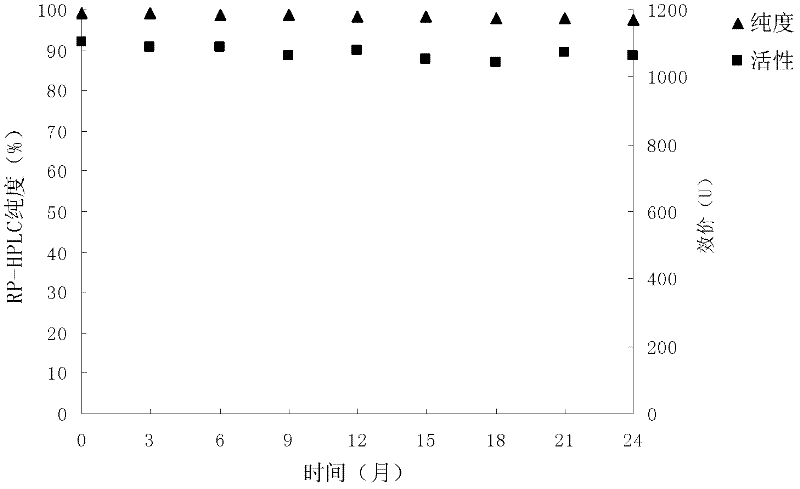
[0055] Embodiment 2: the impact of different carbohydrates on the stability of preparations
[0056] Investigate the influence of different carbohydrates on the stability of freeze-dried preparations according to the following conditions: respectively with 25mM, pH7.3Tris-HCl, 0.2mM ZnCl 2 Prepare the buffer solution with a concentration of 10% sucrose, lactose, glucose and trehalose; take 5ml of 2000U / ml carboxypeptidase G2 stock solution, add 3ml of the above sugar solution and 2ml of buffer solution to obtain: 1000U of carboxypeptidase G2 activity / ml, a solution with a sugar content of 3%. A total of 10ml of each sample was prepared, divided into vials at a rate of 1.1ml / branch, freeze-dried, placed at 25°C for 20 days, and then sampled for RP-HPLC analysis and activity determination.
[0057]
[0058] The above results show that the addition of sugar is beneficial to the maintenance of stability, among which sucrose has the best protective effect, and the activity and...
Embodiment 3
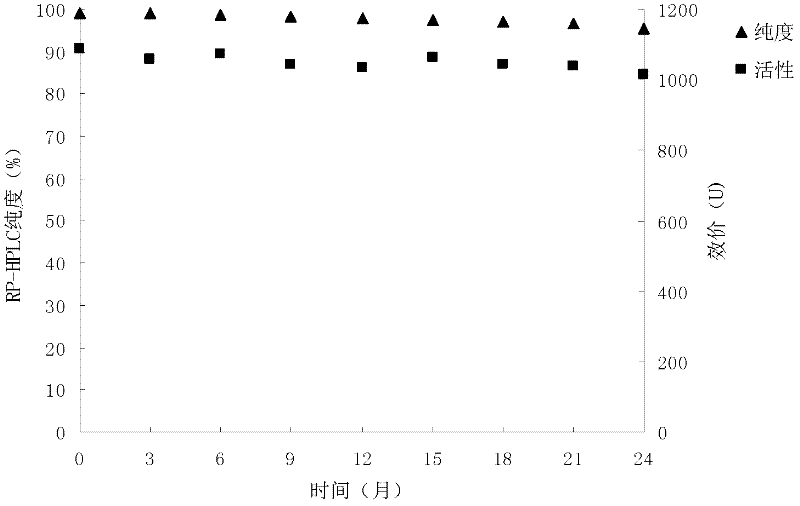
[0064] Embodiment 3: the impact of different pH buffering agents on formulation stability
[0065] The final purified sample (containing 25mM Tris-HCl pH7.3) was ultra-filtered with a membrane with a molecular weight cut-off of 10kD, respectively, with 25mmol / L disodium hydrogen phosphate-citric acid buffer solution with a pH of 4.0-6.0, pH7.0 ~pH9.0 25mmol / L Tris-HCl buffer solution for buffer solution exchange. Add 3% mannitol and 1% sucrose into the sample, prepare 10ml of each sample, put 1.1ml / branch into vials, freeze-dry, and take samples after 20 days at 25°C for RP-HPLC analysis and activity Determination.
[0066]
[0067] The above results show that the pH7.0-8.0 buffer system maintains the best drug activity. According to existing research reports, the optimal pH of the enzyme protein is 7.3, which is close to the physiological pH environment of the human body. Therefore, pH 7.3 is the best buffer pH for the preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com