Medical biological tissue structure and preparation method and special device of medical biological tissue structure
A technology of biological tissue and special equipment, which is applied in the field of tissue and organ manufacturing, can solve the problems of inability to meet the requirements of complex tissue and organ precursor manufacturing, single cells cannot be accurately positioned, coded, and the randomness of cell concentration is large, and it can achieve quantitative spraying technology Maximization and accuracy, elimination of mutual interference, and convenient manufacturing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
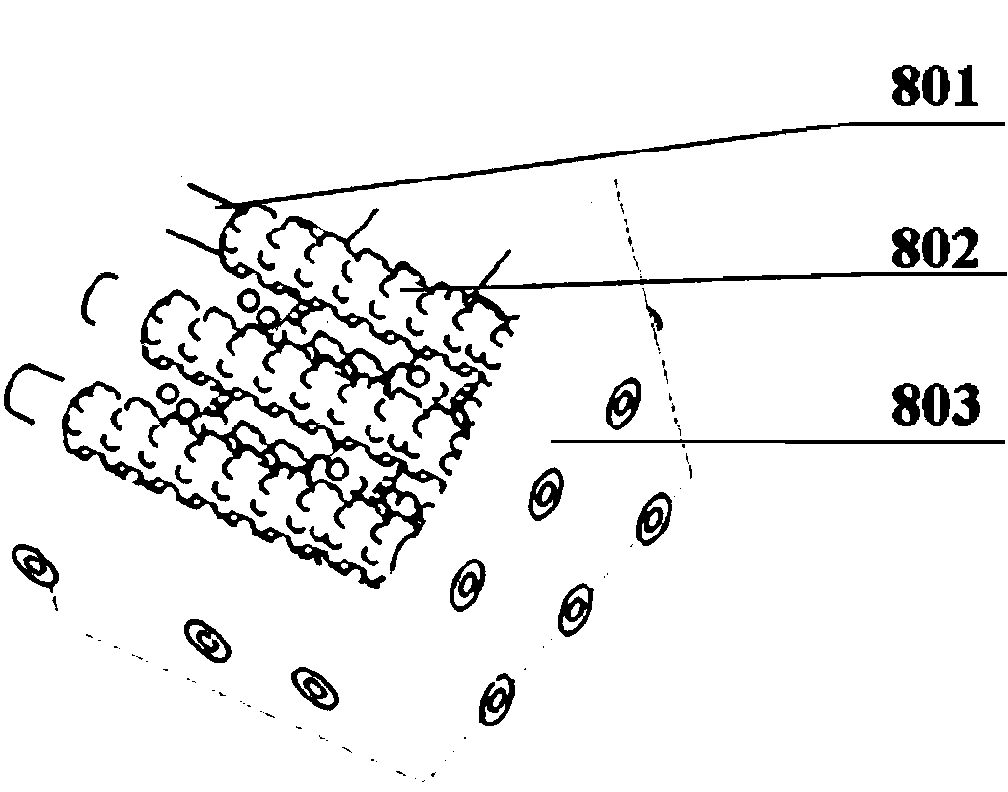
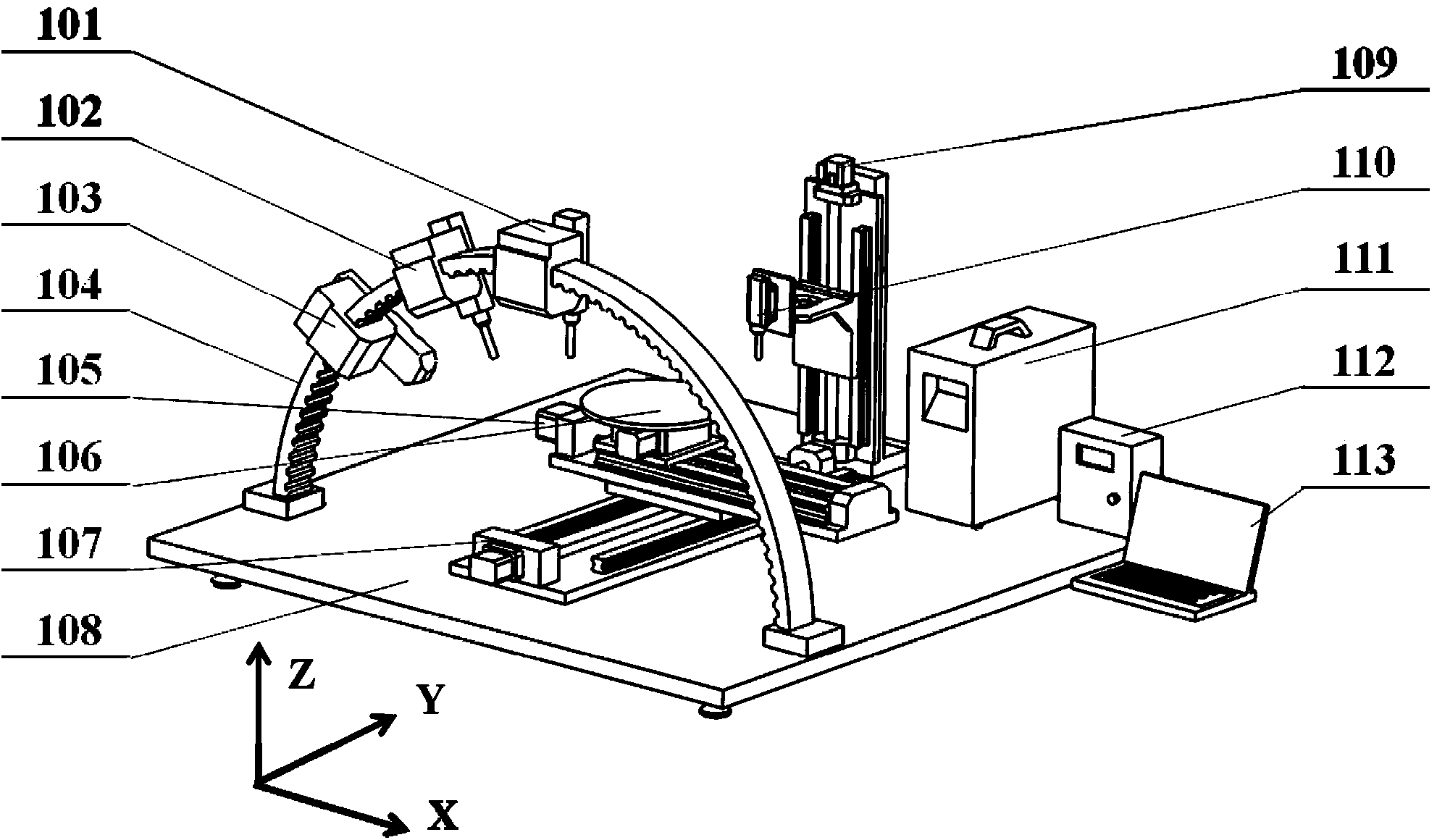
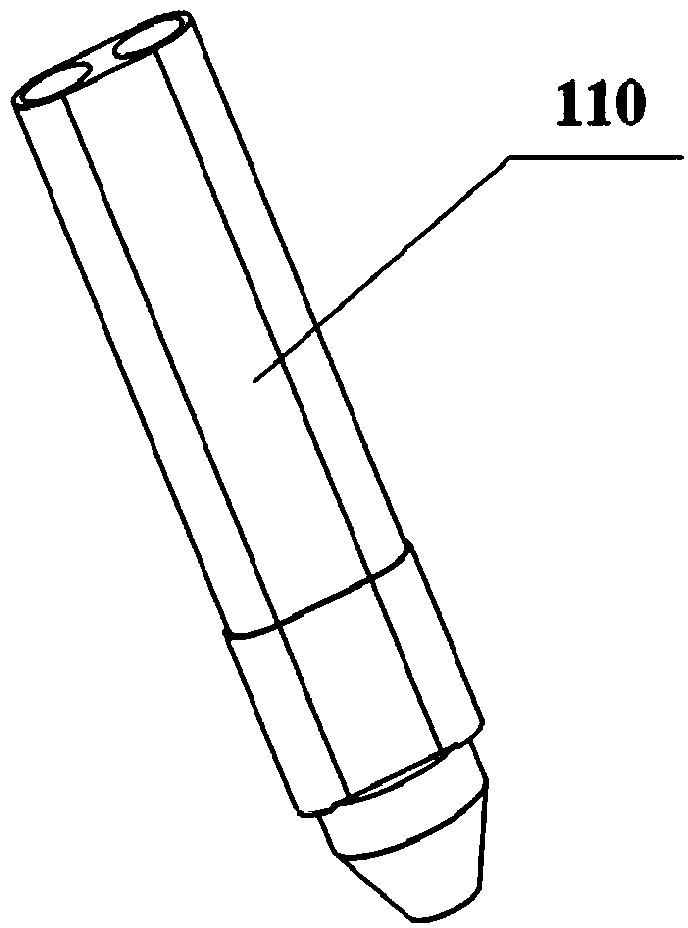
[0074] Embodiment 2: The natural polymer solution and the cross-linking agent are sodium alginate solution and calcium chloride solution. The concentration of sodium alginate solution is 5% (w / v), and the concentration of calcium chloride solution is 1% (w / v). Mix the endothelial cells into the sodium alginate solution at a density of 1 x 10 5 individual / mL. The sodium alginate solution and the calcium chloride solution containing endothelial cells are respectively loaded into two different component syringes of the hollow tube forming nozzle 110 for standby.
[0075] Cardiomyocytes and Schwann cells were extracted from patients. The above cells and sodium alginate solution were mixed. The cardiomyocyte density was 1 x 10 6 cells / mL, the Schwann cell density was 1×10 4 individual / mL. Fill the cell-containing polymer solutions into corresponding syringes for later use.
[0076] PLGA is dissolved in tetraethylene glycol solution to make a solution with a concentration of ...
Embodiment 3
[0085] Example 3: The natural polymer solution and the cross-linking agent are calcium hydrogen phosphate dihydrate (DCPD) and calcium hydroxide (1M disodium hydrogen phosphate) solution. The weight percentages of DCPD and calcium hydroxide in 1M disodium hydrogen phosphate solution are 20% and 10% (w / v) respectively. Put DCPD and calcium hydroxide solution into two different component syringes of the hollow tube forming nozzle 110 for standby.
[0086] Osteoblasts and adipose stem cells are extracted from patients. Mix the above cells with 5% (w / v) gelatin (PBS) solution. The density of osteoblasts was 1×10 5 cells / mL, the density of adipose-derived stem cells was 1×10 2 individual / mL. Add endothelial cell growth factor (10ng / mL) to fill the cell-containing polymer solutions into corresponding syringes for later use.
[0087] Dissolving PU in tetraethylene glycol solution to make a solution with a concentration of 10% (w / v), put it in the spray solution syringe of protec...
Embodiment 4
[0095] Example 4: The natural polymer solution and the cross-linking agent are polylactic-glycolic acid (PLGA) solution and water, respectively. Wherein the weight percent of PLGA in 1.4 dioxane is 20% (w / v). The PLGA solution and water are respectively loaded into two different component syringes of the hollow tube forming spray head 110 for standby.
[0096] Prepare 5% (w / v) gelatin (PBS) and 1% (w / v) fibrinogen solution and mix them, add 0.01% (w / v) heparin and osteoblast growth factor respectively, and put them into corresponding syringes for later use.
[0097] Dissolving PU in tetraethylene glycol solution to make a solution with a concentration of 20% (w / v), put it in the spray solution syringe of protective film nozzle 101 for subsequent use.
[0098] Driven by the X-direction motion device 105 and the Y-direction motion device 107, the rotary forming table 106 moves to the set position below the hollow tube forming nozzle, and the hollow tube forming nozzle 110 extru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com