A solid-state electrolyte, a method for making the same, and an all-solid-state lithium battery
A solid electrolyte, lithium battery technology, applied in solid electrolytes, non-aqueous electrolytes, secondary batteries, etc., can solve the problems of poor battery cycle performance, complex process conditions, difficult to control, etc., and achieve low grain resistance, ion conductivity, etc. High rate, improve the effect of the interface
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
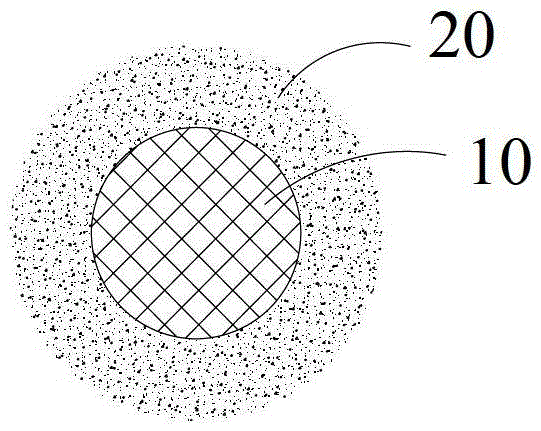
[0022] refer to figure 2 , the present invention also relates to a preparation method of a solid electrolyte, the preparation method comprising the following steps:
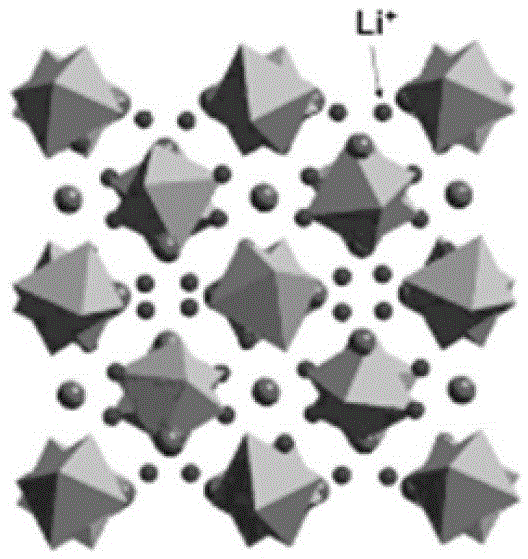
[0023] Step 1: Provide a kind of matrix 10, described matrix is garnet type fast ion conductor Li 7 m 3 Zr 2 o 12 or Li 5 Ta 3 m 2 o 12 , where M is one or more of La, Al, Sr, Sc, Cr, Ba, Fe, Mo, Y;
[0024] Step 2: Coating a layer of surface modification layer 20 on the surface of the substrate 10 by one of radio frequency magnetron sputtering, pulsed laser deposition, and electron beam evaporation, and the surface modification layer 20 is amorphous silicic acid One of lithium, lithium sulfate, or lithium tungstate, such as figure 2 shown.
[0025] Generally, the thickness of the surface modification layer is 0.1 nm to 500 nm. The lithium silicate is Li 2 SiO 3 , Li4 SiO 4 , Li 8 SiO 6 , Li 2 Si 2 o 5 , Li 6 Si 2 o 7 or Li 2 Si 5 o 11 .
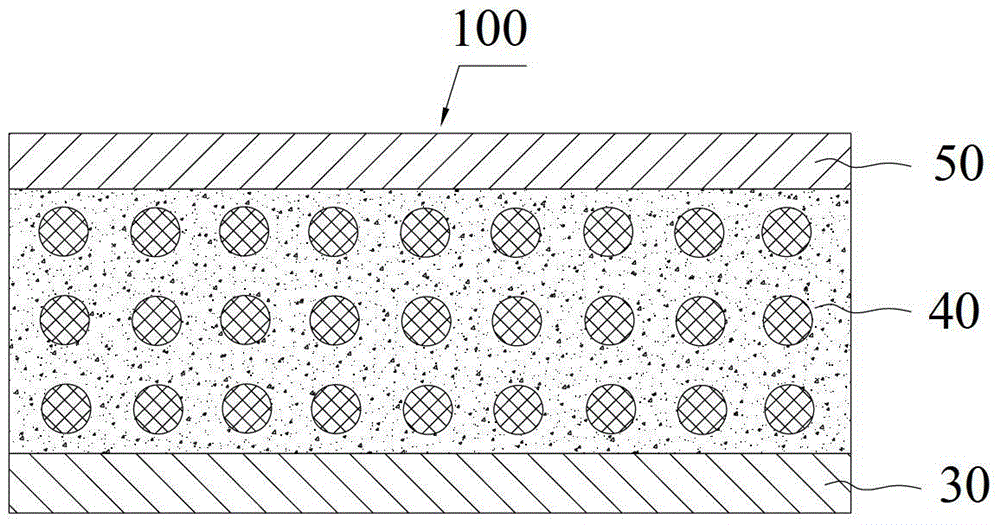
[0026] Such as image 3 As shown, the all-s...
Embodiment 1
[0030] (1) Preparation of garnet-type fast ion conductors (Li 7 La 3 Zr 2 o 12 electrolyte)
[0031] The Li 7 La 3 Zr 2 o 12 The electrolyte is synthesized by a solid-phase method. First, provide the raw material lithium hydroxide (LiOH is produced by AlfaAesar, content >99.9%), treat the lithium hydroxide in an oven at 200°C for 6 hours, and cool to room temperature; add lithium with a molar ratio of 10% to the raw material hydrogen Lithium oxide to compensate for the loss of lithium in the annealing process; add lithium hydroxide, lanthanum oxide (the La 2 o 3 Produced by AlfaAesar, the content is >99.99%; it is calcined at 900°C for 24 hours), and zirconia (the ZrO 2 It is produced by Aldrich company, the content is >99%), the powder is dissolved in isopropanol, and passed through zirconia ball milling in air atmosphere for 12 hours. This process is annealed at 900 ° C, and then passed through zirconia in air atmosphere. Ball milling for 12 hours, this process is...
Embodiment 2
[0038] (1) Preparation of garnet-type fast ion conductors (Li 7 La 3 Zr 2 o 12 electrolyte)
[0039] The Li 7 La 3 Zr 2 o 12 Electrolytes were also synthesized by solid-phase methods. The preparation method is the same as in Example 1.
[0040] (2) Preparation of garnet-type fast ion conductors with a modified layer on the surface
[0041] The above garnet-type Li 7 La 3 Zr 2 o 12 The electrolyte is placed on the substrate as a substrate, and lithium silicate (Li 4 SiO 4 ) as a target, placed in the radio frequency magnetron sputtering equipment, through the method of radio frequency magnetron sputtering in the garnet-type Li 7 La 3 Zr 2 o 12 A layer of lithium silicate (Li 4 SiO 4 ), and finally the surface is coated with lithium silicate (Li 4 SiO 4 ) garnet-type fast ion conductor material, that is: composite garnet-type fast ion conductor, marked as A2. In this example, lithium silicate (Li 4 SiO 4 ) has a thickness of 10 nm on the surface of the g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com