Tetrandrine derivatives and preparation method thereof, and application of tetrandrine derivatives in preparing anti-tumor medicines
A technology for tetrandrine and derivatives is applied in the fields of tetrandrine derivatives and their preparation and application in the preparation of antitumor drugs, and can solve the problems of unsatisfactory therapeutic effect of liver cancer and the like, and achieves simple preparation method and improved performance. Proliferation inhibitory activity, high product purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
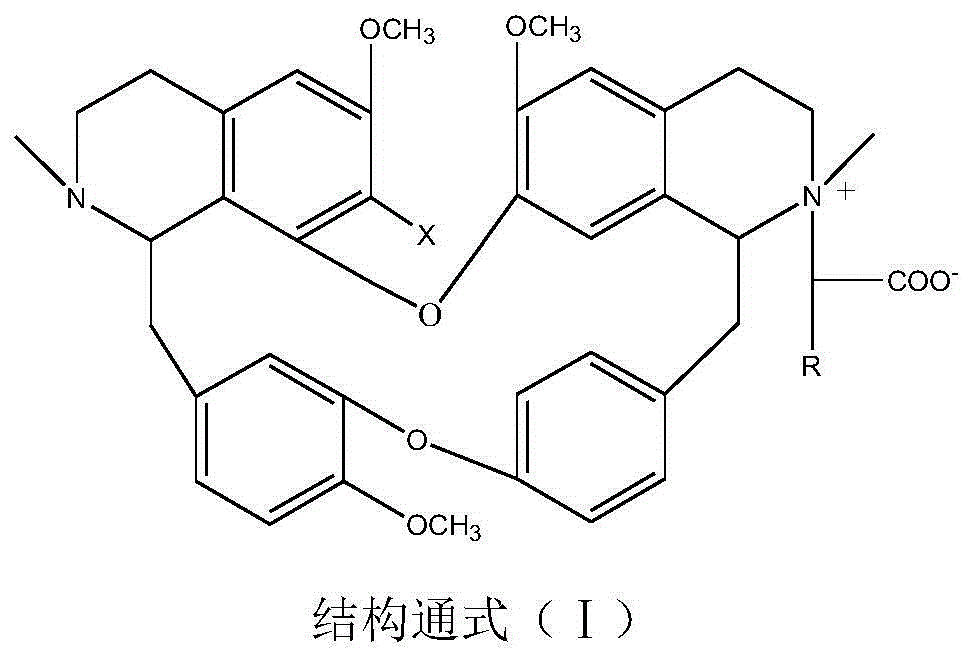
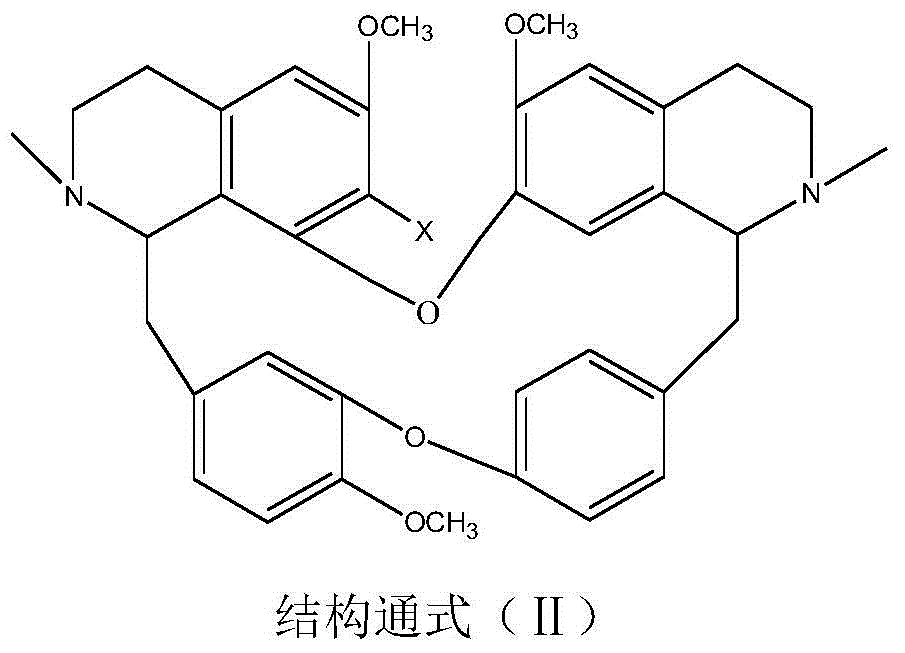
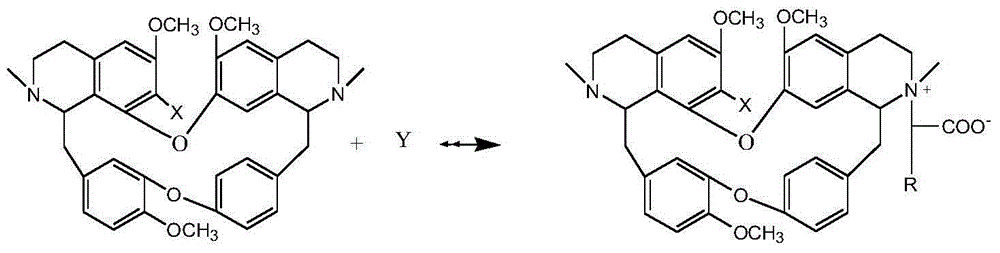
[0036] Weigh 5.92g of bisbenzylisoquinoline (in general formula II, X=H), 2.50g of sodium 2-chloroacetate and 1.05g of potassium carbonate, add them into a 500mL three-necked flask, dissolve in 200mL of ethanol, stir and heat to reflux, and Insulate and react for 8 hours, evaporate the solvent under reduced pressure, cool down to room temperature, neutralize to neutral with 10% HCl, add 50mL of water and extract with chloroform for 3 times (60mL×3), extract with anhydrous Na 2 SO 4 After drying for 8 hours, TLC tracked the separation and purification process of the reaction and the product, recovered chloroform, and dried the solid at 60°C for 4 hours to obtain 2.84 g of a yellow powder product with a yield of 41.33% and a purity of 98.51% (HPLC). The melting point of the target product: 153-154°C, time-of-flight mass spectrum: M / e (326.1574), and the molecular formula is C 39 h 43 o 7 N 2 Cl, 13 C NMR (75MHz, DMSO-d 6 ): δ20.65(C-4), 23.24(C-4’), 35.67(C-15), 49.70(C-15...
Embodiment 2
[0038] Weigh 6.10g of 7-hydroxybisbenzylisoquinoline (in general formula II, X=OH), 10.00g of sodium 2-bromoacetate and 0.50g of potassium carbonate, dissolve them in 150mL of n-butanol, and add them to a 500mL three-necked flask , stirred and heated to boiling, and kept stirring for 4 hours, distilled off the solvent under reduced pressure, cooled to room temperature, neutralized to neutral with 5% HBr, added 50 mL of water and extracted 3 times with acetone (60 mL×3), traced the relationship between the reaction and the product by TLC Separation and purification process, extract with anhydrous Na 2 SO 4 After drying for 8 hours, acetone was recovered, and the solid was dried at 60°C for 4 hours to obtain 3.54 g of a yellow powder product with a yield of 47.35% and a purity of 97.23% (HPLC). The melting point of the target product: 152-153°C, time-of-flight mass spectrum: M / e (334.1548), and the molecular formula is C 39 h 43 o 8 N 2 Br, 13 CNMR shows a carbonyl peak at...
Embodiment 3
[0040] Weigh 6.26g of 7-chlorobisbenzylisoquinoline (in general formula II, X=Cl), 3.00g of sodium 2-chloroacetate, and 1.20g of anhydrous sodium carbonate, dissolve them in 100mL of water, add them to a 500mL three-necked flask, Stir and freeze to 5°C, keep warm and stir to react for 24 hours, after rising to room temperature, neutralize with 10% HCl to neutral, distill out water under reduced pressure until the liquid volume is reduced to half, crystallize at room temperature for 8 hours, filter, and track the separation of reaction and product by TLC During the purification process, the obtained solid was dried at 60°C for 4 hours to obtain 2.75 g of a yellow powder product with a yield of 38.11% and a purity of 96.55% (HPLC). The melting point of the target product: 162-163°C, time-of-flight mass spectrum: M / e (343.1379), and the molecular formula is C 39 h 42 o 7 N 2 Cl 2 , 13 CNMR showed a carbonyl peak at δ=167.04, X-ray single crystal diffraction, namely compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com