Freeze-dried preparation for treating gynecological diseases as well as preparation method and application thereof
A technology of gynecological diseases and freeze-dried preparations, which is applied in the medical field, can solve the problems of lack of gynecological diseases, long-term cure, and female diseases, and achieve the effect of strong antibacterial and anti-inflammatory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
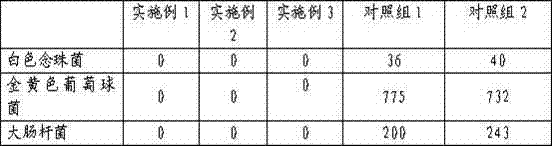
Examples
Embodiment 1
[0030] Freeze-dried powder of the present invention is configured as:
[0031] Weigh 10g of human beta defensin and 600g stabilizer, add 10000ml of injection grade water, mix well, filter and sterilize, fill in 3.2ml in a vial with a half stopper and put it into a vacuum freeze dryer for 15 hours to freeze dry.
[0032] The process conditions of vacuum freeze-drying are as follows: pre-freeze the product, lower the temperature to -50°C, cool down for 4 hours, evacuate to 0.3mbar, heat and sublimate and dry for 15 hours, until the water in the product is completely sublimated .
[0033] Wherein the stabilizer formula is: 200g glycine, 300g protein hydrolyzate, 100g (10mM) phosphoric acid.
[0034] The configuration of basic liquid in the present embodiment is:
[0035] Basic liquid formula:
[0036] Hyaluronic Acid 21.22g
[0037] Methylpropanediol 106.1g
[0038] Honey Extract 318.3g
[0039] Sodium PCA 159.15g
[0040] Dissolved Protease 265.25g
[0041] 1,2 hexanedio...
Embodiment 2
[0047] Freeze-dried powder of the present invention is configured as:
[0048] Weigh 50g of human beta defensin and 650g of stabilizer, add 10000ml of injection grade water, mix well, filter and sterilize, fill in 3.2ml in a vial with a half-stopper and put it into a vacuum freeze dryer for 25 hours to freeze-dry.
[0049] The process conditions of vacuum freeze-drying are: pre-freeze the product, lower the temperature to -50°C, cool down for 6 hours, vacuumize to 0.3mbar, heat and sublimate and dry for 20 hours, until the water in the product sublimates completely .
[0050] Wherein the stabilizer formula is: 200g trehalose, 300g sodium thiosulfate, 150g (15mM) sodium carbonate.
[0051] The configuration of basic liquid in the present embodiment is:
[0052] Basic liquid formula:
[0053] Hyaluronic Acid 26.75g
[0054] Honey Extract 214g
[0055] Sodium PCA 321g
[0056] Dissolving Protease 321g
[0057] 1,2 hexanediol 53.5g
[0058] Oat Beta Glucan 214g
[0059] ...
Embodiment 3
[0063] The freeze-dried powder is configured as follows: 100g human beta defensin, 600g stabilizer, add 10000ml injection grade water, mix evenly, filter and sterilize, fill in 3.2ml vials and half-stopper them, then put them into a vacuum freeze dryer to freeze-dry 35h. The process conditions of vacuum freeze-drying are: pre-freeze the product, lower the temperature to -50°C, cool down for 8 hours, vacuumize to 0.3mbar, heat and sublimate and dry for 25 hours, until the water in the product sublimates completely .
[0064] Wherein the stabilizer formula is: 200g sucrose, 300g vitamin D, 100g (10mM) potassium citrate.
[0065] The configuration of basic liquid in the present embodiment is:
[0066] Basic liquid formula:
[0067] Hyaluronic Acid 32.1g
[0068] Methylpropanediol 267.5g
[0069] Honey Extract 374.5g
[0070] Sodium PCA 214g
[0071] Dissolving Protease 321g
[0072] Oat Beta Glucan 374.5g
[0073] Soy Isoflavones 428g
[0074] Deionized water 10000ml. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com