Dikaryotic cobalt complex and preparation method thereof
A technology of cobalt complexes and crystals, applied in cobalt organic compounds, chemical instruments and methods, luminescent materials, etc., can solve problems affecting the development and application of metal-organic coordination polymers, unsatisfactory fluorescence properties, etc., and achieve simple synthesis methods Easy operation and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] ①. Weigh 0.25g cobalt chloride hexahydrate, 0.2g 2-(3-pyridyl)benzimidazole, 0.2g 1,3-benzenedicarboxylic acid and 15mL water in a 50mL flask, place the flask on a magnetic stirrer and stir Mix for 30 minutes.
[0035] ②. Transfer the uniformly mixed solution to a 25mL autoclave.
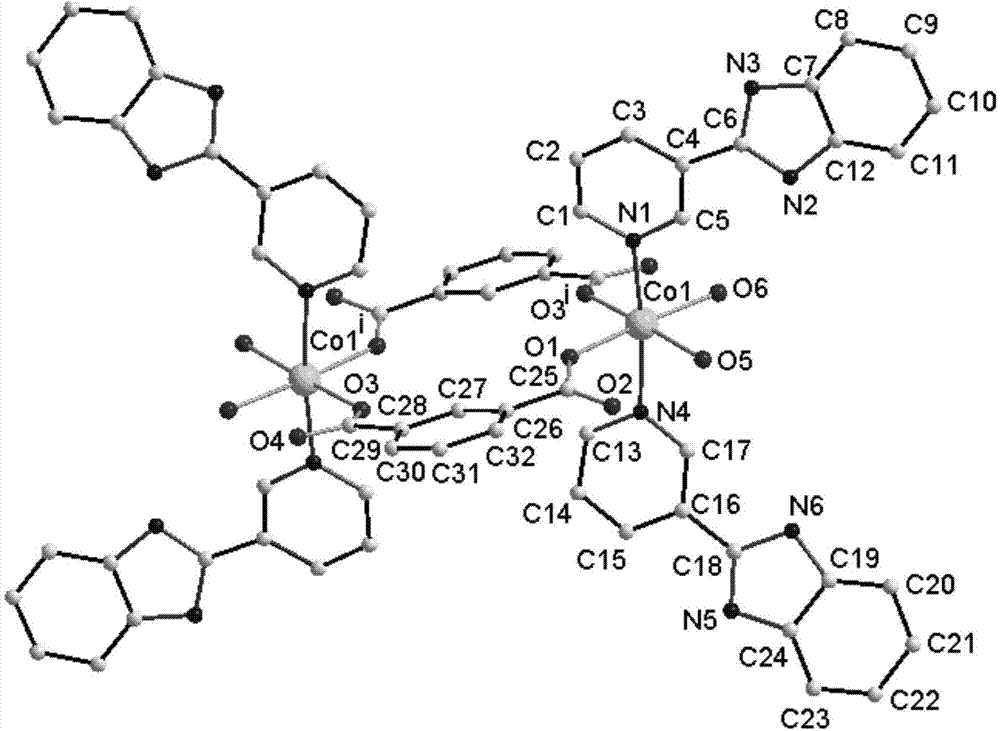
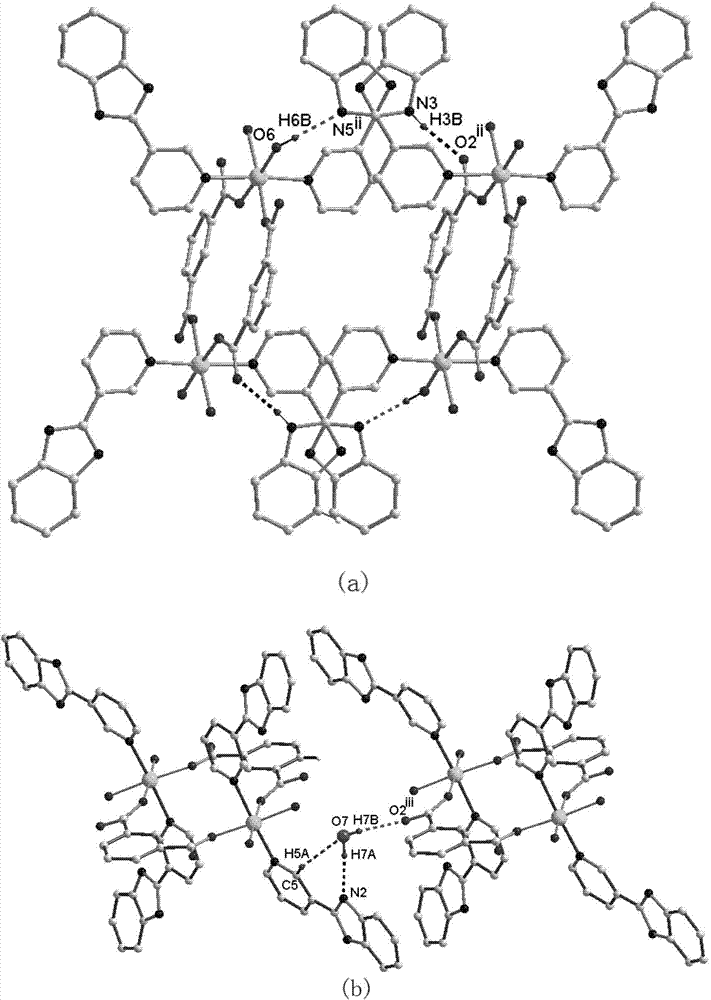
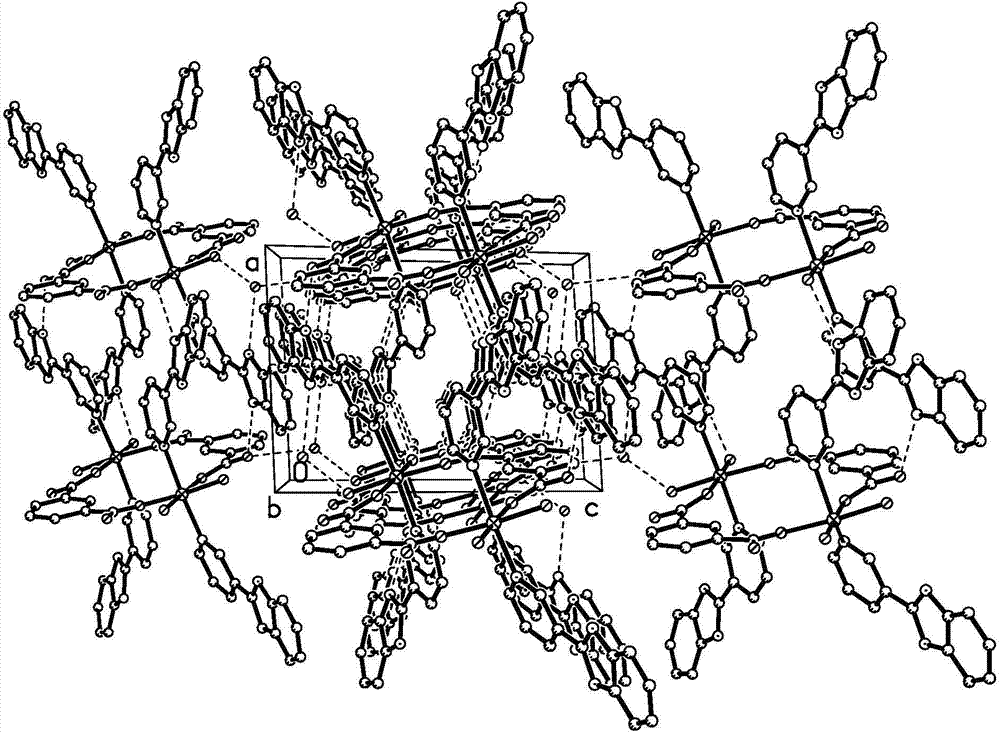
[0036] ③. Put the autoclave into an oven, heat it from room temperature to 170 °C at a rate of 1 °C / min, keep it at this temperature for 72 hours, and then lower it to room temperature at a rate of 5 °C / h. The solid-liquid separation is as follows: Figure 1-3 as shown, Figure 4-6 The dinuclear cobalt complex shown in the curve has a yield of about 65%.
[0037] The crystal structure of the target product prepared in this embodiment was determined by a single crystal diffractometer, and the results were as follows:
[0038] Crystal data:
[0039]
[0040] Crystal bond length data
[0041] Co(1)-O(3) i 2.077(2) Co(1)-O(5) 2.125(2) Co(1)-O(1) 2.096(2) Co(1)-N(1...
Embodiment 2
[0047] ①. Weigh 0.20g of cobalt chloride hexahydrate, 0.15g of 2-(3-pyridyl)benzimidazole, 0.15g of 1,3-phthalic acid and 12mL of water in a 50mL flask, and place the flask on a magnetic stirrer for stirring Mix for 20 minutes.
[0048] ②. Transfer the uniformly mixed solution to a 25mL autoclave.
[0049] ③. Put the autoclave into an oven, heat it from room temperature to 170 °C at a rate of 1.5 °C / min, keep it at this temperature for 60 hours, and then lower it to room temperature at a rate of 3 °C / h. The solid-liquid separation is as follows: Figure 1-3 shown, approximately Figure 4-6 The dinuclear cobalt complex shown in the curve has a yield of about 65%.
Embodiment 3
[0051] ①. Weigh 0.30g of cobalt chloride hexahydrate, 0.25g of 2-(3-pyridyl)benzimidazole, 0.25g of 1,3-phthalic acid and 18mL of water in a 50mL flask, and place the flask on a magnetic stirrer to stir Mix for 10 minutes.
[0052] ②. Transfer the uniformly mixed solution to a 25mL autoclave.
[0053] ③. Put the autoclave into an oven, heat it from room temperature to 170°C at a rate of 2°C / min, keep it at this temperature for 80 hours, then lower it to room temperature at a rate of 4°C / h, and obtain the following solid-liquid separation: Figure 1-3 shown, approximately Figure 4-6 The dinuclear cobalt complex shown in the curve has a yield of about 64%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com