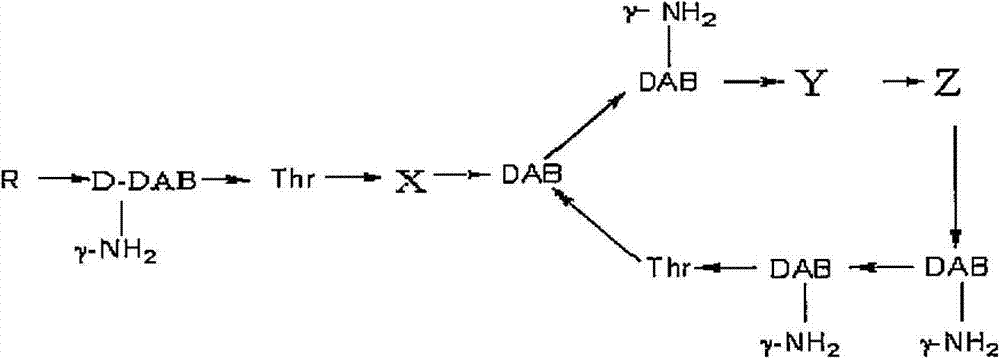
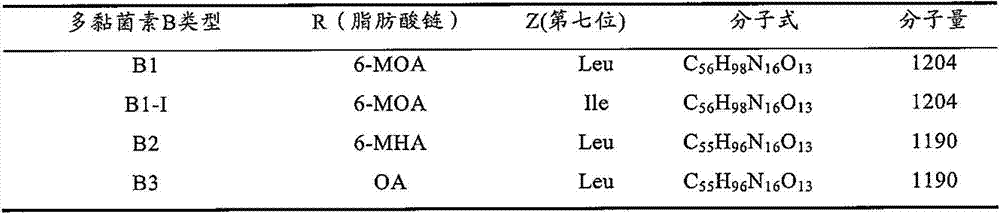
Method for separation purification of polymyxin B1 from polymyxin B mixed component
A technology of polymyxin and mixed components, which is applied in the field of medicine and can solve the problems of high equipment requirements, poor separation effect, and difficulty in large-scale preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Take polymyxin B sulfate sample, add water to dissolve and configure 50mg / ml aqueous solution.
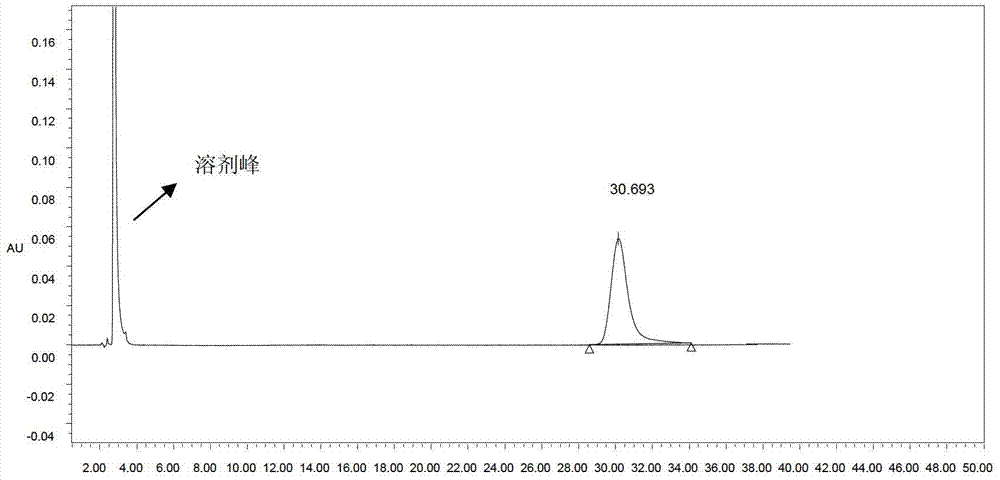
[0031] Pack PS30-300 (Suzhou Nan Microbe Technology Co., Ltd., particle size of 30 μm) chromatography column bed 30ml, after equilibrating 3 times column volume with acidic aqueous solution (formic acid to adjust pH2.3) containing 8% acetonitrile (V / V), Get a 10ml sample and put it on the column, and then elute it with an acidic aqueous solution containing 8% acetonitrile (formic acid adjusts the pH to 2.3). The purity of colistin B1 was 99.2%, and the yield was 63%.
Embodiment 2
[0033] Take polymyxin B sulfate sample, add water to dissolve and configure 10mg / ml aqueous solution.
[0034]Pack 30ml of PS25-300 (Suzhou Nan Microbe Technology Co., Ltd., particle size 25μm) chromatography column bed, equilibrate 3 times column volume with acidic aqueous solution containing 9% acetonitrile (adjust pH 2.0 with formic acid), take 30ml sample and put it on the column , and then eluted with an acidic aqueous solution containing 9% acetonitrile (formic acid to adjust the pH to 2.0). After the eluate was analyzed by HPLC, the part of the polymyxin B1 peak purity greater than 98% was collected, and the obtained polymyxin B1 had a purity of It was 99.5%, and the yield was 60%.
Embodiment 3
[0036] Take polymyxin B sulfate sample, add water to dissolve and configure 100mg / ml aqueous solution.
[0037] Install 30ml of PS30-300 chromatography column bed, equilibrate 3 times column volume with acidic aqueous solution containing 15% ethanol (adjust pH 2.3 with formic acid), take 3ml sample on the column, and then use acidic solution containing 15% ethanol (V / V) Aqueous solution (formic acid adjusted to pH 2.3) was eluted, and after the eluate was analyzed by HPLC, the part of polymyxin B 1 peak purity greater than 98% was collected, and the obtained polymyxin B 1 had a purity of 98.6% and a yield of 45% %.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com