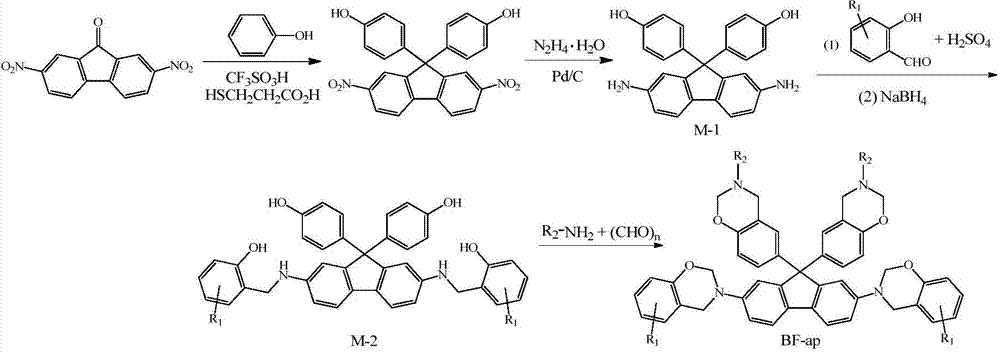
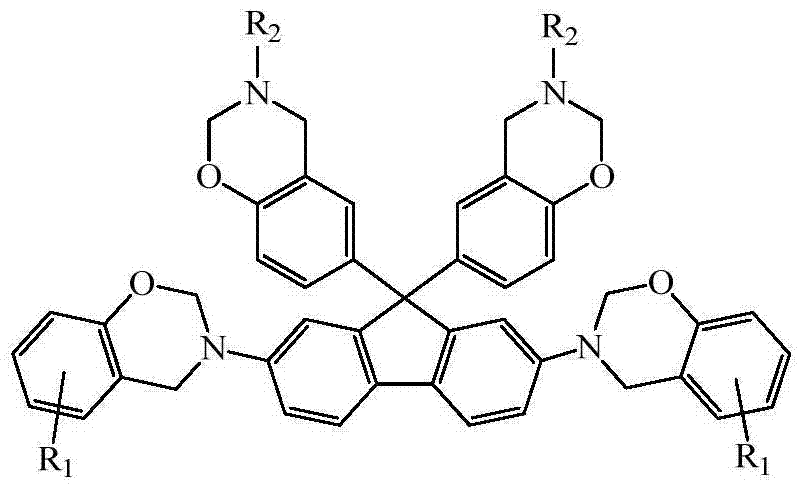
N-semi aromatic hydrocarbyl diamine-bisphenol tetrafunctional fluorene-based benzoxazine and preparation method thereof
A semi-aromatic hydrocarbon-based diamine and four-functionality technology, which is applied in organic chemistry and other fields, can solve the problems of unstable aliphatic aminooxazine ring, inability to obtain the target product, easy ring-opening reaction, etc., and achieve the total yield of the product Improvement, excellent thermal stability, low mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Synthesis of 2,7-diamino-9,9-bis-(4-hydroxyphenyl)fluorene
[0029] Add 0.01mol 2,7-dinitro-9-fluorenone, 0.08mol phenol, 0.005mol trifluoromethanesulfonic acid and 0.001mol 3-mercaptopropionic acid to a three-necked flask with a stirring rotor, a condenser tube and a thermometer in sequence, Stirring was started, the reaction temperature was controlled at 55°C, and the reaction time was 3 hours. After the reaction, the product was washed with 20% ethanol solution for 3 to 5 times, and finally dried in vacuum to obtain 2,7-dinitro-9,9-bis -(4-hydroxyphenyl)fluorene, yield 86.8%; 0.05mol2,7-dinitro-9,9-bis-(4-hydroxyphenyl)fluorene, 100mL absolute ethanol and 2 grams of palladium carbon catalyst Add it into the above-mentioned three-necked flask, raise the reaction temperature to 85°C under stirring, then add dropwise 0.47mol80% hydrazine hydrate solution, finish the reaction after 36h, filter while it is hot, and remove most of the ethanol by distilling the filtrate...
Embodiment 2
[0037] Except that the ethylamine in the synthesis step (3) was changed to n-butylamine, the solvent 40mL dioxane and 10mL chlorobenzene were changed to 40mL toluene and 10mL chlorobenzene, and the reaction time was changed from 24h to 12h, other conditions were the same as in Example 1 , other conditions are the same as in Example 1, and finally the N-semiaromatic hydrocarbon base diamine-bisphenol type tetrafunctional fluorenyl benzoxazine monomer derived from 4-methyl salicylaldehyde-butylamine is obtained, T m It was 108°C, and the yield was 62.7%.
[0038] 1 H NMR: 6.58~7.79 (m, 18H, Ar-H), 5.33 and 4.73 (d, 8H, O-CH 2 -N), (d,4H,O-CH 2 -N), 4.55 and 3.94 (d, 8H, Ar-CH 2 -N), 2.68(t,4H,N-CH 2 -), 2.30 (s, 6H, Ar-CH 3 ), 1.55 and 1.34 (m, 8H, -CH 2 -), 0.92 (t, 6H, -CH 3 ); FT-IR: 1495, 1384, 1366 and 1320, 1251 and 1223, 1155, 1076 and 1035, and 928-953.
[0039] Curing condition is the same as embodiment 1, the T of polybenzoxazine g : 318,T 5 :336,Y c : 39%. ...
Embodiment 3
[0041] Except that the 4-methyl-2-hydroxybenzaldehyde in the synthesis step (2) was changed to 2-hydroxybenzaldehyde, the heating and reflux time was changed from 6h to 8h, the ethylamine in the synthesis step (3) was changed to octylamine, and the solvent Change 40mL dioxane and 10mL chlorobenzene into 45mL chloroform and 5mL chlorobenzene, change the reaction temperature from 110°C to 62°C, change the reaction time from 24h to 48h, other conditions are the same as in Example 1, and finally obtain salicylaldehyde- Octylamine-derived N-semiaromatic diamine-bisphenol-type tetrafunctional fluorenylbenzoxazine, T m It was 66°C, and the yield was 72.3%.
[0042] 1 H NMR: 6.53~7.81 (m, 20H, Ar-H), 5.33 and 4.85 (d, 8H, O-CH 2 -N), 4.56 and 3.85 (d, 8H, Ar-CH 2 -N), 2.69 (t, 4H, N-CH-), 1.51 and 1.27 (m, 24H, -CH 2 -), 0.87(t,6H,-CH 3 ); FT-IR: 1497, 1379, 1368 and 1321, 1271 and 1225, 1155, 1076, 928-953 and 724 (long chain aliphatic hydrocarbon C-H rocking vibration).
[004...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tg | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com