Crystal form of icaritin compound, drug containing crystal form and application of crystal form
A technology for alcaradine and its compounds, which is applied in the fields of medical preparations containing active ingredients, drug combinations, organic chemistry, etc., and can solve problems such as poor stability, drug quality risks, and the coexistence of multiple crystal forms of pure alcaradine, etc. problem, to achieve the effect of long shelf life and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of Alcoradine by Enzymatic Hydrolysis of Commercial Epimedium Extract
[0052] The epimedium extract in this example was purchased from Shaanxi Jiahe Plant Chemical Co., Ltd., with a trade name of "Epimedium extract", which contained icariin with a mass fraction of 90%.
[0053] Step 1: Preparation of Alcoradine by Enzymatic Hydrolysis
[0054] 80 g of commercially available Epimedium extract, which contains 90% icariin by mass fraction, is dispersed in 2.0 L of disodium hydrogen phosphate-potassium dihydrogen phosphate buffer solution of pH 5.2 at a concentration of 1 mol / L , add 0.6L of ethanol, 1400g of RAPIDASE pectinase, a total of 1.4L into a 5L reactor, and carry out enzymatic hydrolysis at a reaction temperature of 50°C. The specific conditions are shown in Table 1:
[0055] Table 1 Enzymolysis conditions
[0056] Epimedium extract g
Enzyme amount L
Ethanol L
Buffer L
temperature reflex
80
1.4
0.6
2.0
...
Embodiment 2
[0064] The crystals obtained in Example 1 were continuously dried at 80° C. until the weight of the crystals no longer changed. The weight of the crystals after drying at 80° C. was weighed, which was reduced relative to the weight of the crystals in Example 1.
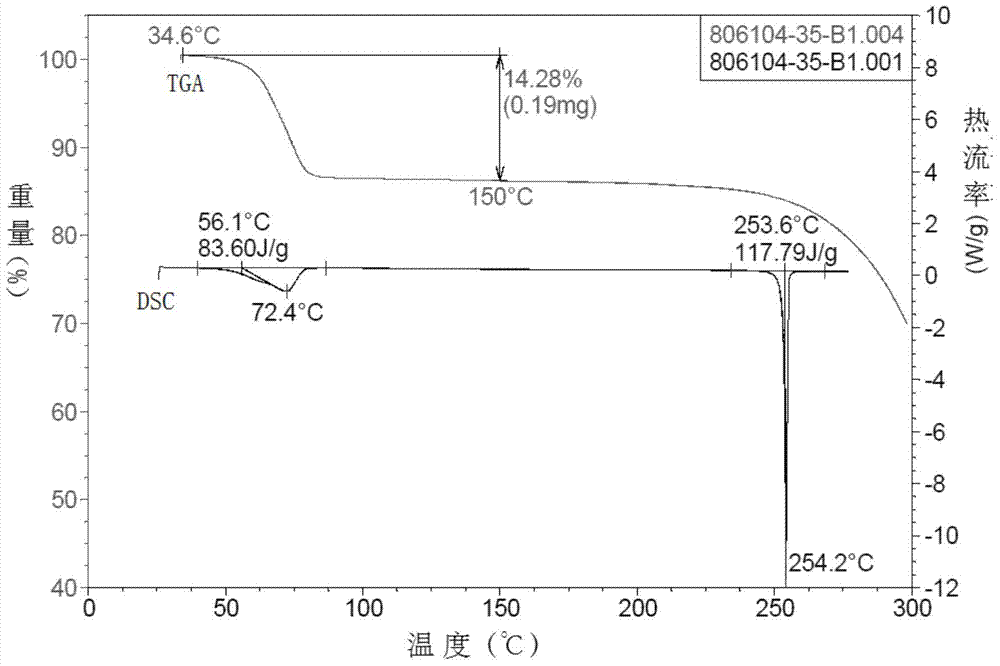
[0065] Carry out thermal gravimetric analysis and differential scanning thermal analysis to the crystal obtained according to the above method, obtain as follows Figure 4 The graph shown.
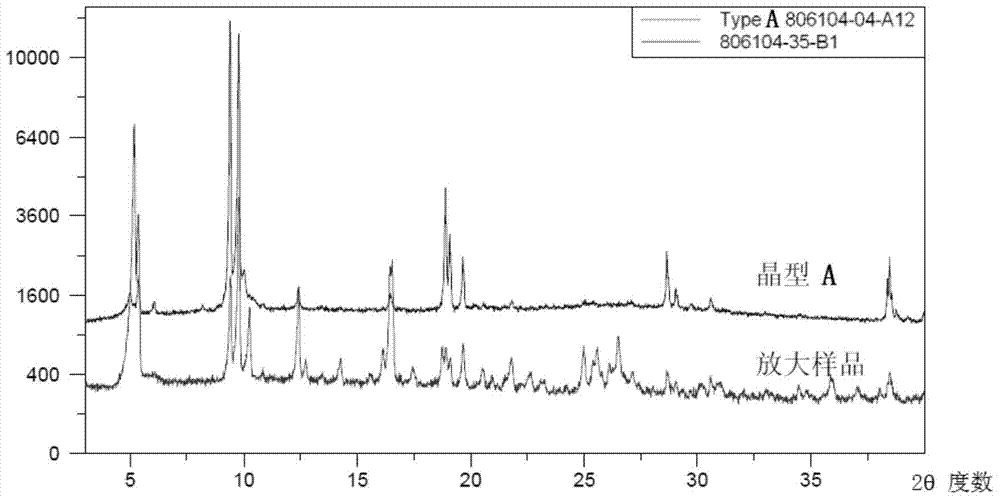
[0066] exist Figure 4 Among them, differential scanning thermal analysis: that is, there is a melting endothermic peak at 253.0°C in the DSC curve, and the melting enthalpy is 137.29J / g. And in the thermogravimetric analysis curve, that is, when the sample is heated to 150° C. in TGA, the weight loss is 0.35%, so it can be considered from the above curve that the crystal obtained in this example is a single crystal form, that is, crystal form B.
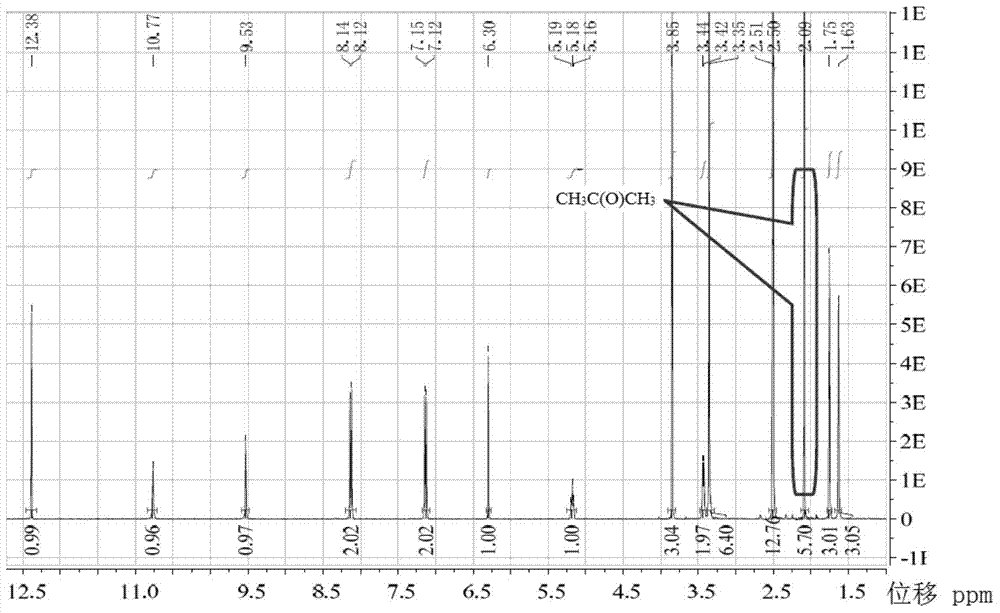
[0067] Figure 5 Represent the nuclear magnetic resonance spectrum figure of cr...
Embodiment 3
[0070] Weigh 15 mg of the solvent-free crystalline form of Acradine obtained in Example 2 into a 1.5 mL vial, and add 1.2 mL of methanol solvent to obtain a suspension. The suspension was stirred at 20°C for 4 days, and the obtained crystals were collected by centrifugation, and subjected to thermogravimetric analysis and differential scanning thermal analysis to obtain the following: Figure 7 The graph shown. It can be seen from the spectrum that when the sample is heated to 100°C, the weight loss is 5.5%, the desolvation endothermic peaks appear at 89.0°C and 107.3°C, and there is a melting endotherm at 253.8°C, and the melting enthalpy is 117.51J / g.
[0071] pass Figure 8 It can be seen from the NMR images of the Figure 5 In contrast, a peak with an integral value of 1.04 appears at a displacement of 3.17ppm. According to the displacement, it is inferred that this peak is a characteristic peak representing methanol, because it is known that the methoxyl group in the st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com