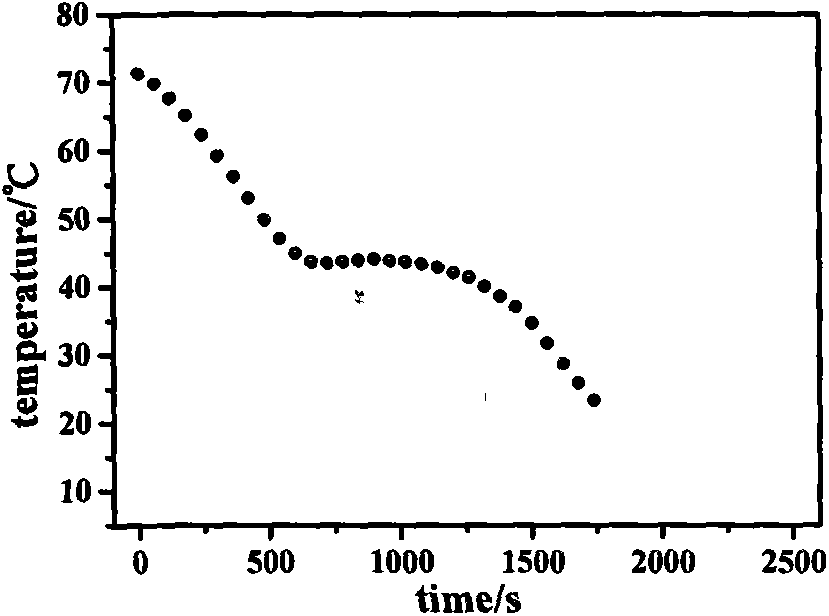
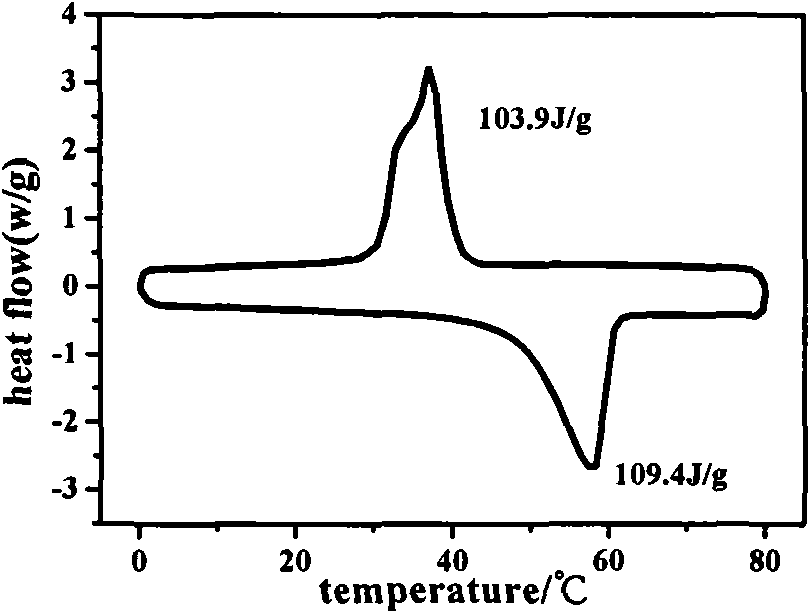
Synthetic method of phase change material polytetramethylene glycol amine aldehyde condensation crosslinking copolymer
A technology of polybutylene glycol amine aldehyde, phase change energy storage material, applied in heat exchange materials, chemical instruments and methods, chemical industry and other directions, can solve problems such as easy leakage, reduction of crystallization enthalpy, reduction of phase change enthalpy, etc. , to achieve the effect of low condition requirements, simple process and few reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The quality of each raw material component that prepares polytetramethylene glycol 4000-acetaldehyde-syrylenetriamine cross-linked copolymer is as follows:
[0022] Polytetramethylene glycol 4000 40g
[0023] Triamine 5g
[0024] Acetaldehyde solution (w%>40%) 10g
[0025] The synthetic method of polytetramethylene glycol 4000-acetaldehyde-sylene triamine condensation cross-linked copolymer is as follows:
[0026] (1) Add 40g of polytetramethylene glycol 4000, 10g of acetaldehyde solution, and 5g of pyrenetriamine into 100g of water, stir slowly until completely dissolved, at a temperature of 50°C, add acetic acid dropwise to adjust the pH to 6, and react for 180min;
[0027] (2) Put the polytetramethylene glycol 4000-acetaldehyde-symylene triamine condensation cross-linked copolymer precursor mixed solution obtained in (1) in a thermostat at 100°C until the water is completely removed, and about 46g of polytetramethylene glycol can be obtained. Butylene Glycol 4000-...
Embodiment 2
[0032] Prepare the quality of each raw material component of polytetramethylene glycol 10000-acetaldehyde-phenylenediamine cross-linked copolymer as follows:
[0033] Polytetramethylene glycol 10000 40g
[0034] Phenylenediamine 5g
[0035] Acetaldehyde solution (w%>40%) 10g
[0036] The synthetic method of polytetramethylene glycol 10000-acetaldehyde-phenylenediamine condensation cross-linked copolymer is as follows:
[0037] (1) Add 40g of polytetramethylene glycol 10000, 10g of acetaldehyde solution, and 5g of phenylenediamine into 100g of water, stir slowly until completely dissolved, at a temperature of 50°C, add acetic acid dropwise to adjust the pH to 4, and react for 180min;
[0038] (2) Put the polytetramethylene glycol 10000-acetaldehyde-phenylenediamine condensation cross-linked copolymer precursor mixed solution obtained in (1) in a thermostat at 120°C until the water is completely removed, and about 46g of polytetramethylene glycol can be obtained Diol 10000-acet...
Embodiment 3
[0043] Prepare the quality of each raw material component of polytetramethylene glycol 2000-acetaldehyde-butylene diamine cross-linked copolymer as follows:
[0044] Polytetramethylene glycol 2000 50g
[0045] Butylenediamine 5g
[0046] Acetaldehyde solution (w%>40%) 10g
[0047] The synthetic method of polytetramethylene glycol 2000-acetaldehyde-butylene diamine condensation cross-linked copolymer is as follows:
[0048] (1) Add 50g of polytetramethylene glycol 2000, 10g of acetaldehyde solution, and 5g of butanediamine into 130g of water, stir slowly until completely dissolved, at a temperature of 50°C, add acetic acid dropwise to adjust the pH to 5, and react for 180 minutes;
[0049] (2) Put the polytetramethylene glycol 2000-acetaldehyde-butylene diamine condensation cross-linked copolymer precursor mixed solution obtained in (1) in a thermostat at 100°C until the water is completely removed, and about 56g of polytetramethylene glycol can be obtained Diol 2000-acetald...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com