Fluidized bed catalyst for directly preparing low-carbon olefins from synthesis gas and producing method thereof
A technology of fluidized bed catalysts and low-carbon olefins, applied in the direction of physical/chemical process catalysts, metal/metal oxides/metal hydroxide catalysts, chemical instruments and methods, etc., can solve the problem of low weight selectivity of low-carbon olefins, Catalyst is easy to deactivate, fixed bed reaction is difficult to remove heat, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
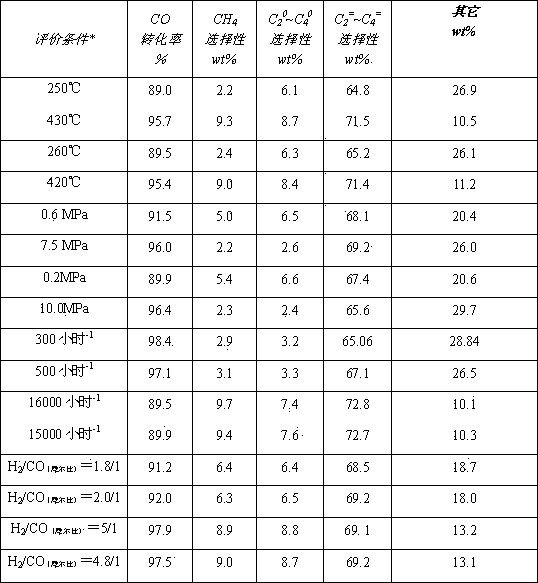
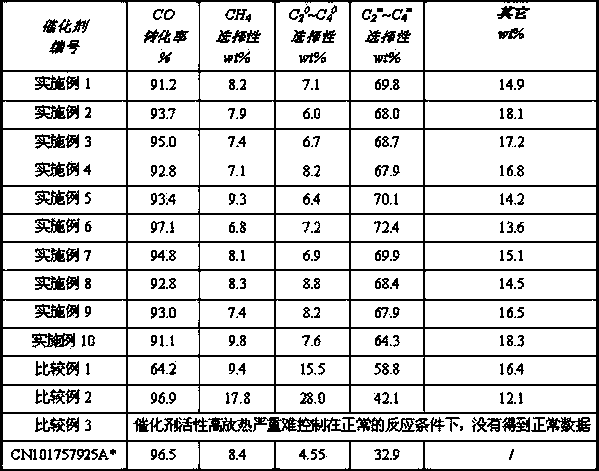
Image
Examples
Embodiment 1
[0029]Dissolve 606.03g of ferric nitrate nonahydrate and 0.88g of ammonium metavanadate in water to form solution I, dissolve 132.40g of ammonium heptamolybdate and 0.24g of scandium nitrate pentahydrate in water to obtain solution II, and mix solutions I and II Obtain solution III; dissolve 87.98g of zinc nitrate hexahydrate in water to make a solution, and flow it with 200g of ammonia water with a mass concentration of 6% (into the same container dropwise at a uniform rate according to the proportion of just complete reaction) to obtain zinc hydroxide precipitation , fully wash the precipitate with deionized water to obtain zinc hydroxide; add zinc hydroxide to solution Ⅲ and mix and beat to obtain slurry Ⅳ, dissolve 0.08g of potassium hydroxide in water to obtain solution Ⅴ and then add it to the slurry Mix and beat in IV to obtain slurry VI, then adjust the pH value of the slurry to 5 with ammonia water, obtain the required catalyst slurry (solid content 15wt%) after full s...
Embodiment 2
[0032] Dissolve 606.03g of ferric nitrate nonahydrate and 569.13g of titanium tetrachloride in water to form solution I, dissolve 3.64g of bismuth nitrate pentahydrate and 86.19g of yttrium nitrate hexahydrate in water to obtain solution II, and mix solutions I and II Obtain solution III; dissolve 932.86g of zirconium nitrate pentahydrate in water to make a solution, and flow it with 2500g of ammonia water with a mass concentration of 6% (into the same container dropwise at a uniform rate according to the proportion of just complete reaction) to obtain zirconium hydroxide Precipitate, fully wash the precipitate with deionized water to obtain zirconium hydroxide; add zirconium hydroxide to solution III, mix and beat to obtain slurry IV, dissolve 20.23g of cesium hydroxide in water to obtain solution V and then add it to the slurry Mix and beat material IV to obtain slurry VI, then adjust the pH value of the slurry to 1 with ammonia water, and obtain the required catalyst slurry ...
Embodiment 3
[0035] Dissolve 606.03g of ferric nitrate nonahydrate and 35.2g of ammonium metavanadate in water to form solution I, dissolve 218.40g of bismuth nitrate pentahydrate and 71.83g of yttrium nitrate hexahydrate in water to obtain solution II, and mix solutions I and II Obtain solution Ⅲ; take 960.2g of zirconium nitrate solution and flow it with 2580g of ammonia water with a mass concentration of 6% (into the same container dropwise at a uniform speed according to the proportion of just complete reaction) to obtain zirconium hydroxide precipitation, fully deionized water Wash the precipitate to obtain zirconium hydroxide; add zirconium hydroxide to solution III, mix and beat to obtain slurry IV, dissolve 6g of sodium hydroxide in water to obtain solution V, then add it to slurry IV, mix and beat to obtain slurry Ⅵ, adjust the pH value of the slurry to 3 with ammonia water, obtain the required catalyst slurry (solid content 35wt%) after full stirring, and spray the slurry to shape...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com