Preparation method for carbon-coated nano-titanium dioxide electrode material
A titanium dioxide and nano-electrode technology, which is applied in the preparation/purification of titanium dioxide and carbon, titanium oxide/hydroxide, etc., can solve the problems of cumbersome preparation methods and processes, high manufacturing costs, and achieve low prices, moderate conditions and abundant raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
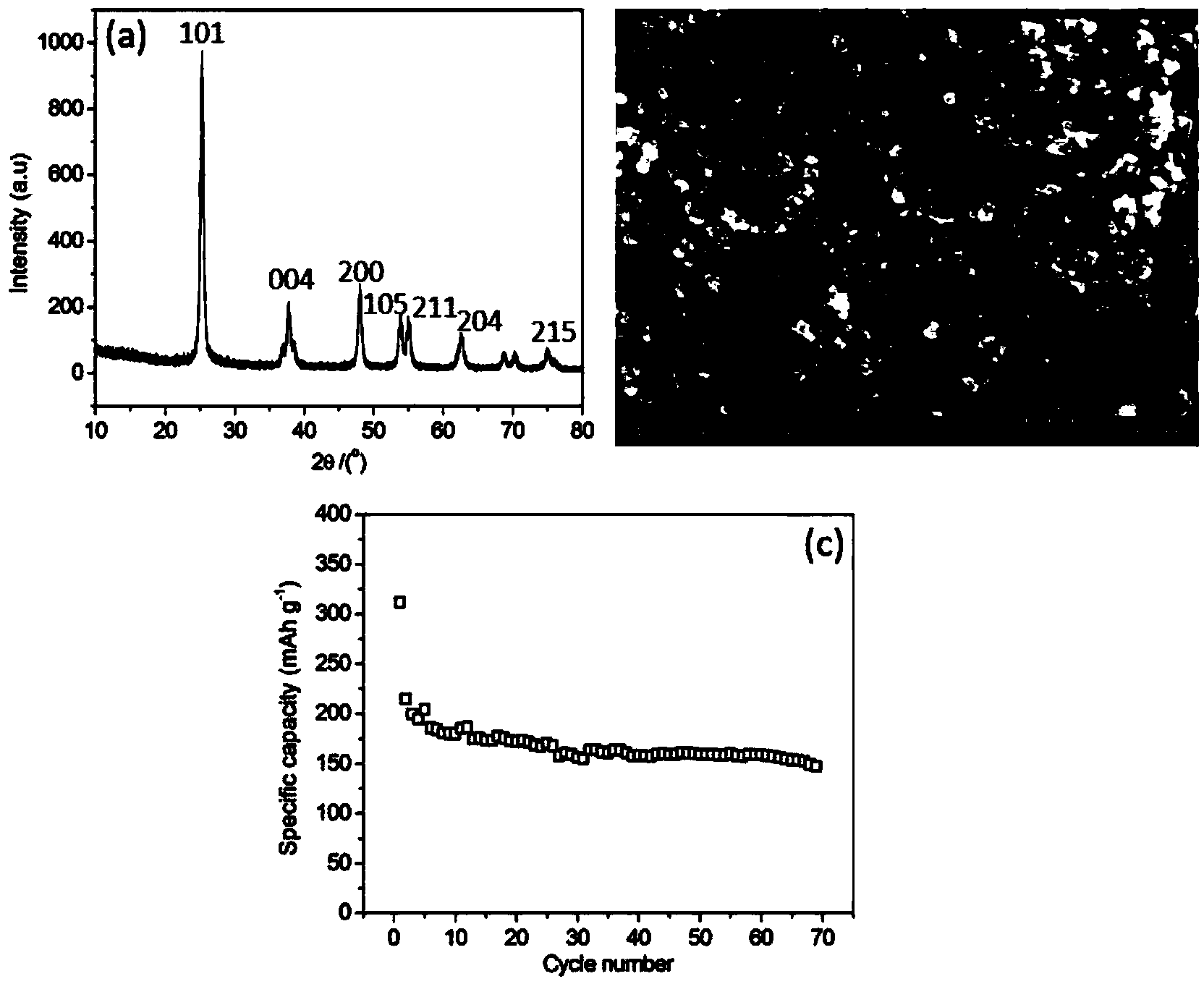
[0020] Weigh titanium sulfate and glucose according to the mass ratio of titanium sulfate and glucose as 1:0.2, respectively dissolve them in deionized water, stir and mix evenly, add the glucose aqueous solution into the titanium sulfate aqueous solution, move it into the hydrothermal reaction kettle, and heat it at 140°C Keep warm for 6 hours. After natural cooling to room temperature, the product was repeatedly washed with deionized water and alcohol, filtered and dried. The dried product was moved into a heating furnace, kept carbonized at 600° C. for 4 hours under an argon protective atmosphere, and cooled naturally to room temperature.
[0021] After drying the product obtained by heating the product at 140°C for 6 hours, it was calcined at 600°C in air for 4 hours, so that the coating on the surface of titanium dioxide was completely oxidized and volatilized. It can be known by measuring the mass loss before and after calcination that the coating on the surface of titan...
Embodiment 2
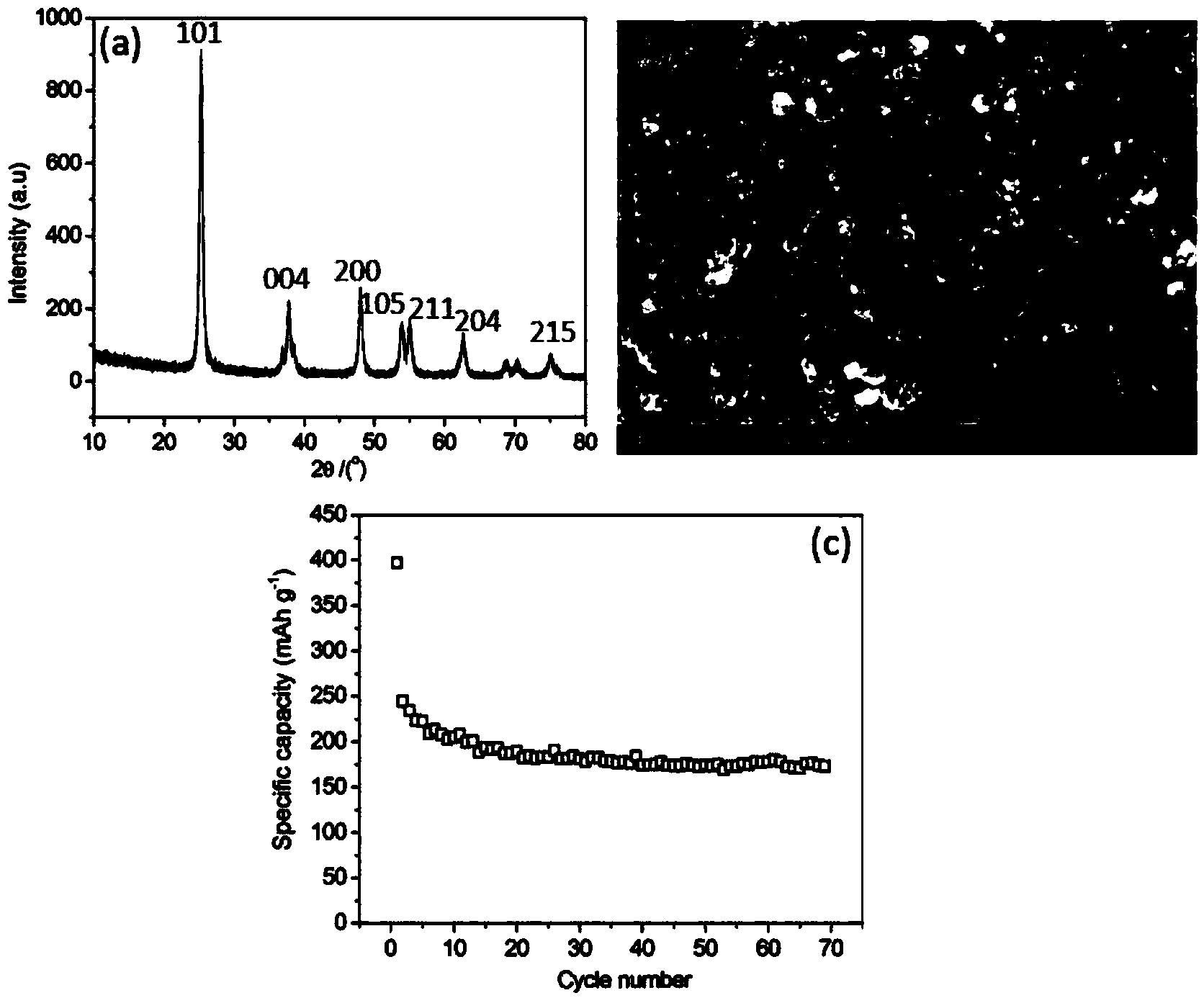
[0023] Weigh titanium sulfate and glucose according to the mass ratio of titanium sulfate and glucose as 1:0.5, respectively dissolve them in deionized water, stir and mix evenly, add the glucose aqueous solution into the titanium sulfate aqueous solution, move it into a hydrothermal reaction kettle, and heat Keep warm for 5 hours. After natural cooling to room temperature, the product was repeatedly washed with deionized water and alcohol, filtered and dried. The dried product was moved into a heating furnace, kept carbonized at 650° C. for 4 hours under an argon protective atmosphere, and cooled naturally to room temperature.
[0024] After drying the product obtained by heating water at 160°C for 5 hours, it was calcined in air at 600°C for 4 hours, so that the coating on the surface of titanium dioxide was completely oxidized and volatilized. It can be known by measuring the mass loss before and after calcination that the coating on the surface of titanium dioxide The pro...
Embodiment 3
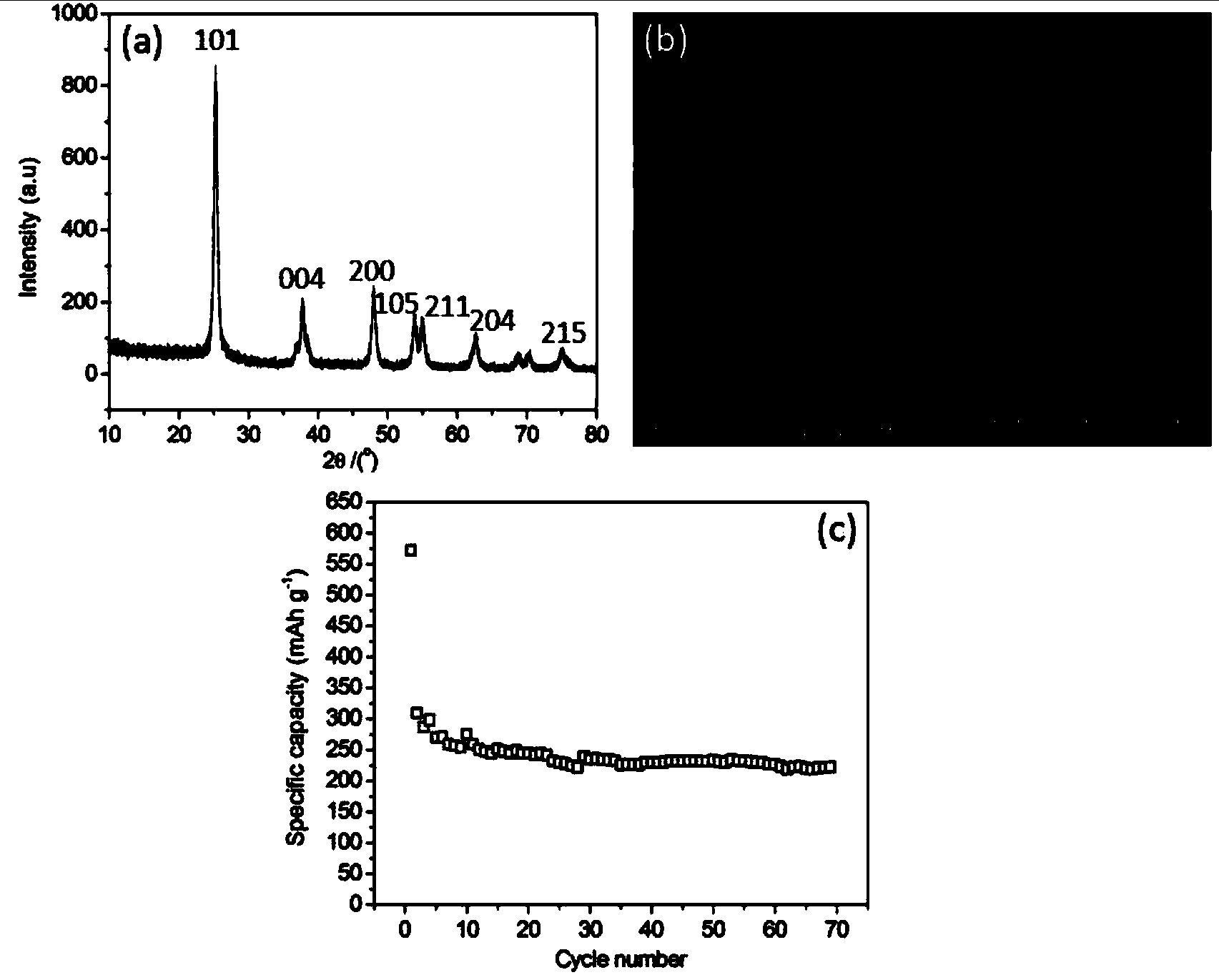
[0026] Weigh titanium sulfate and glucose according to the mass ratio of titanium sulfate and glucose as 1:1.2, respectively dissolve them in deionized water, stir and mix evenly, add the glucose aqueous solution into the titanium sulfate aqueous solution, move it into the hydrothermal reaction kettle, and heat Keep warm for 4 hours. After natural cooling to room temperature, the product was repeatedly washed with deionized water and alcohol, filtered and dried. The dried product was moved into a heating furnace, kept carbonized at 700° C. for 4 hours under an argon protective atmosphere, and cooled naturally to room temperature.
[0027] After drying the product obtained by heating water at 180°C for 4 hours, it was calcined in the air at 600°C for 4 hours, so that the coating on the surface of titanium dioxide was completely oxidized and volatilized. By measuring the mass loss before and after calcination, it can be known that the coating on the surface of titanium dioxide ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com