Novel triazole antifungal compound, pharmaceutical composition and preparation method and application thereof
A compound, triazole technology, applied in the fields of drug synthesis, pharmacology, and pharmacology, can solve the problems of low oral bioavailability, low bioavailability, and the effect of which is greatly affected by food.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
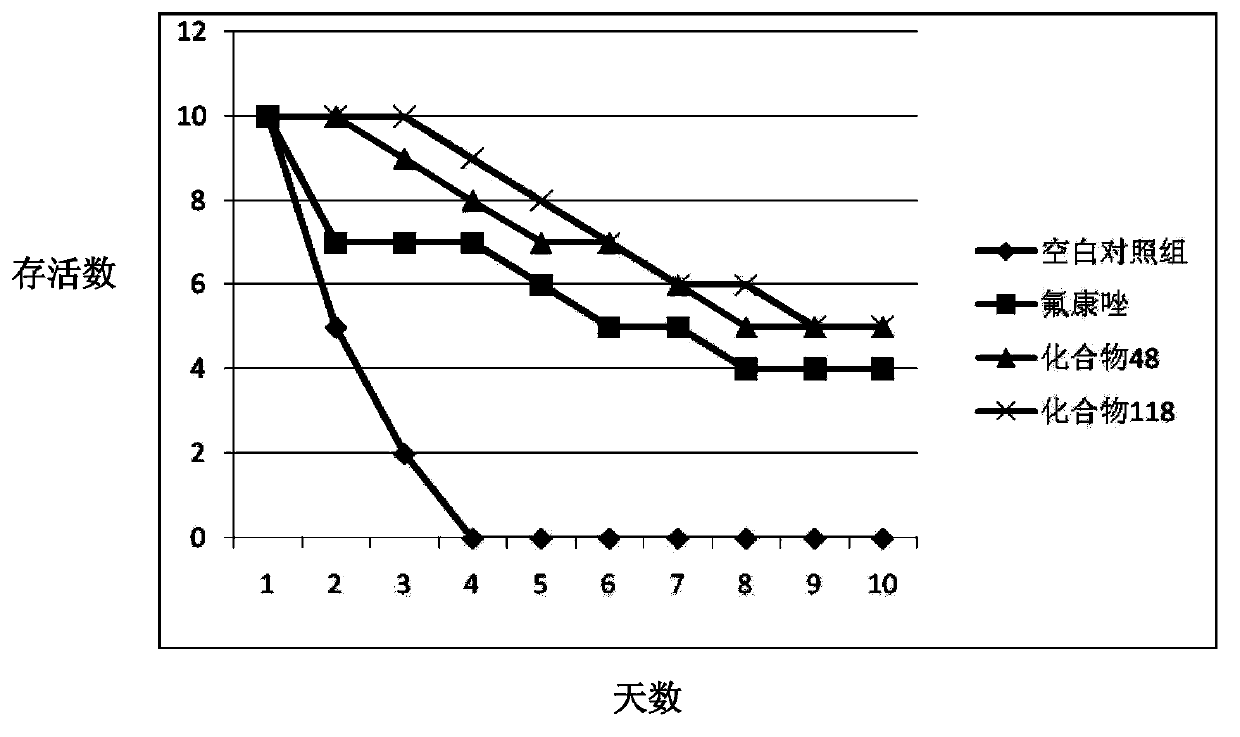
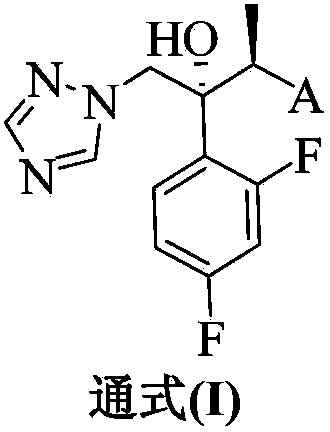
Image
Examples
Embodiment 1
[0137] Example 1 (2R,3R)-2-(2,4-difluorophenyl)-3-(5,6-dihydroimidazo[1,2-a]piperazin-7(8H)-yl)- Preparation of 1-(1H-1,2,4-triazol-1-yl)butan-2-ol (compound 1)
[0138]
[0139] Compound 2A (126.0 mg, 0.48 mmol) was dissolved in 10.0 ml of acetonitrile under Ar gas protection. Compound 3a-1 (118.0mg, 0.96mmol) and lithium perchlorate (102.0mg, 0.96mmol) were added, and the reaction mixture was heated to 80°C for 20 hours, then cooled to room temperature. The reaction mixture was concentrated to dryness under reduced pressure, and the residue was washed with CH 2 Cl 2 dissolved, and the organic phase was washed 3 times with saturated NaCl solution, anhydrous NaCl 2 SO 4 After drying, filtering, and evaporating to dryness, the residue was separated by column chromatography (dichloromethane:methanol=100:1-50:1) to obtain 80.8 mg of white solid compound 1, melting point: 86-88°C, yield 45.0%.
[0140] 1 H NMR (300MHz, CDCl 3 )δ7.86(s,1H),7.79(s,1H),7.48-7.36(m,1H),7.15(...
Embodiment 2
[0141] Example 2 (2R,3R)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)-3-(2-(trifluoromethyl base)-5,6-dihydroimidazo[1,2-a]piperazin-7(8H)-yl)butan-2-ol (compound 2)
[0142]
[0143] Compound 2A (100.0mg, 0.40mmol) was added to compound 3a-2 (152.0mg, 0.80mmol) and lithium perchlorate (85.1mg, 0.80mmol), and prepared in a similar manner to Example 1 to obtain 89.8mg of a white solid Compound 2, melting point: 141-143°C, yield 50.6%.
[0144] 1 H NMR (300MHz, CDCl 3 )δ7.83(s,1H),7.77(s,1H),7.48-7.36(m,1H),7.20(s,1H),6.81-6.66(m,2H),5.01(s,1H),4.96 -4.79(m,2H),4.18-4.00(m,3H),3.95-3.87(m,1H),3.75-3.79(m,1H),3.27(q,J=6.8Hz,1H),2.92-2.71 (m,1H),0.96(d,J=6.8Hz,3H).ESI-MS: 443.1(M+1).
Embodiment 3
[0145] Example 3 (2R,3R)-3-(2-tert-butyl-5,6-dihydroimidazo[1,2-a]piperazin-7(8H)-yl)-2-(2,4- Preparation of difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol (compound 3)
[0146]
[0147] Compound 2A (126.0mg, 0.48mmol) was added to compound 3a-3 (172.0.0mg, 0.96mmol) and lithium perchlorate (102.0mg, 0.96mmol), and prepared in a similar manner to Example 1 to obtain 94.4mg of white Solid compound 3, melting point: 129-131°C, yield 45.6%.
[0148] 1H NMR (300MHz, CDCl 3 )δ7.84(s,1H),7.75(s,1H),7.39(m,1H),6.79-6.67(m,2H),6.62(s,1H),5.02(s,1H),4.97-4.80 (m,2H),4.28-4.21(m,2H),4.12-3.94(m,3H),3.29(q,J=6.8Hz,1H),2.89-2.74(m,1H),1.35(s,9H ),0.94(d,J=6.8Hz,3H).ESI-MS: 431.2(M+1).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com