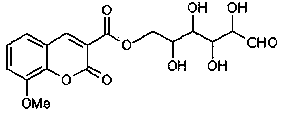
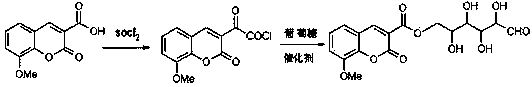Preparation method and application of 8-methoxycoumarin-3-glucose ester formate
A technology of methoxy coumarin and glucose ester, which is applied in the field of preparation of tobacco flavors and fragrances, can solve the problems of short fragrance retention time and limited use, improve smoke and taste, increase production cost, and aroma retention time lengthening effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] a. After adding 0.04mol or 8.8g of 8-methoxycoumarin-3-carboxylic acid and 0.20mol or 23.8g of thionyl chloride into the three-necked flask, stir and reflux at 80°C for 4 hours. After the reaction Rotary evaporation removes excess thionyl chloride to obtain light yellow solid powder 8-methoxycoumarin-3-formyl chloride;
[0025] b. After adding 25ml of dichloromethane, 12.14g (0.12mol) of anhydrous triethylamine and 9.0g (0.05mol) of glucose into the above container, stir the reaction at 40°C for 4h, add 30ml of distilled water after the reaction, and then use 20ml Petroleum ether was extracted 4 times, then dried with anhydrous sodium sulfate (or anhydrous magnesium sulfate or anhydrous calcium chloride) to remove the solvent, and further purified by silica gel column chromatography, wherein the eluent was petroleum ether and acetone at a ratio of 10: The mixed solution prepared in a volume ratio of 1 was purified to obtain 4.36 g of bean-flavored flavor 8-methoxycoum...
Embodiment 2
[0026] Embodiment 2: the difference between this embodiment and embodiment 1 is:
[0027] a. After adding 0.04mol or 8.8g of 8-methoxycoumarin-3-carboxylic acid and 0.08mol or 9.52g of thionyl chloride into the three-necked flask, stir and reflux at 80°C for 4 hours, and rotate after the reaction Evaporate and remove excess thionyl chloride to obtain light yellow solid powder 8-methoxycoumarin-3-formyl chloride;
[0028] b. After adding 25ml of dichloromethane, 12.66g (0.16mol) of anhydrous pyridine and 5.4g (0.03mol) of glucose into the above container, stir the reaction at 30°C for 4 hours, add 30ml of distilled water after the reaction, and then use 20ml of petroleum ether Extract 3 times, then dry with anhydrous magnesium sulfate (or anhydrous sodium sulfate or anhydrous calcium chloride), remove the solvent, and use silica gel column chromatography for further purification, wherein the eluent uses petroleum ether and acetone at a volume ratio of 10:1 Compared with the ...
Embodiment 3
[0029] Embodiment 3: the differences between this embodiment and embodiment 1 are:
[0030] a. After adding 0.04mol or 8.8g of 8-methoxycoumarin-3-carboxylic acid and 0.20mol or 23.8g of thionyl chloride into the three-necked flask, stir and reflux at 80°C for 4 hours, and rotate after the reaction Evaporate and remove excess thionyl chloride to obtain light yellow solid powder 8-methoxycoumarin-3-formyl chloride;
[0031] b. After adding 20ml of dichloromethane, 12.14g (0.12mol) of anhydrous triethylamine and 14.41g (0.08mol) of glucose into the above container, stir and react at 40°C for 3 hours, add 20ml of distilled water after the reaction, and then use 20ml Petroleum ether was extracted 3 times, then dried with anhydrous calcium chloride (or anhydrous magnesium sulfate or anhydrous sodium sulfate) to remove the solvent, and further purified by silica gel column chromatography, wherein the eluent was petroleum ether and acetone at a ratio of 10: The mixed solution prep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com