Method and system for preparing 3-methyl-2-buten-1-ol through ester exchange
A technology of prenol and prenol acetate, which is applied in the field of rectifying separation and preparation of prenol products, can solve the problems of large amount of waste water, difficult treatment, high energy consumption for separation, avoid corrosion, and easily The effect of separation and recovery and simplified process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
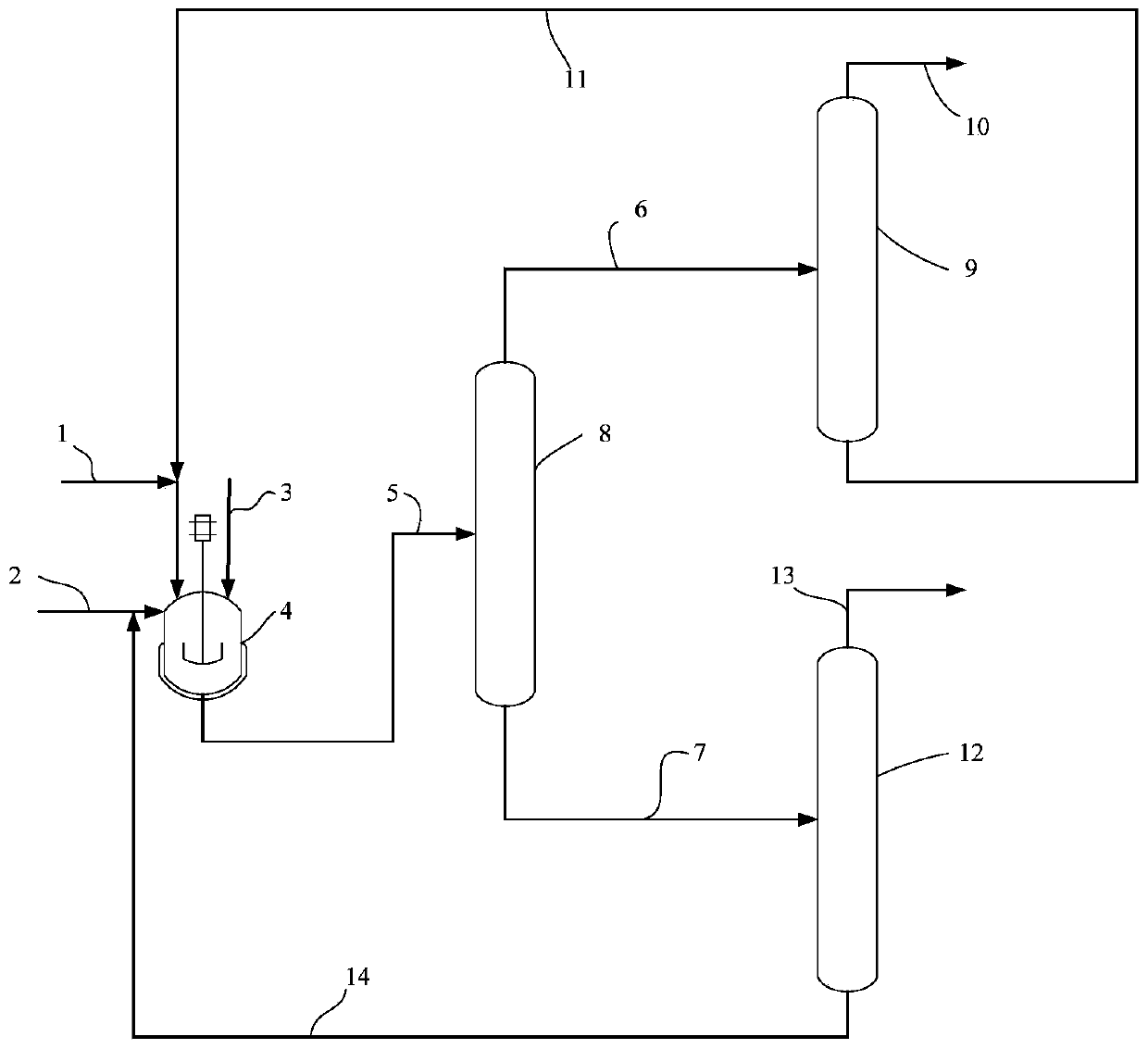
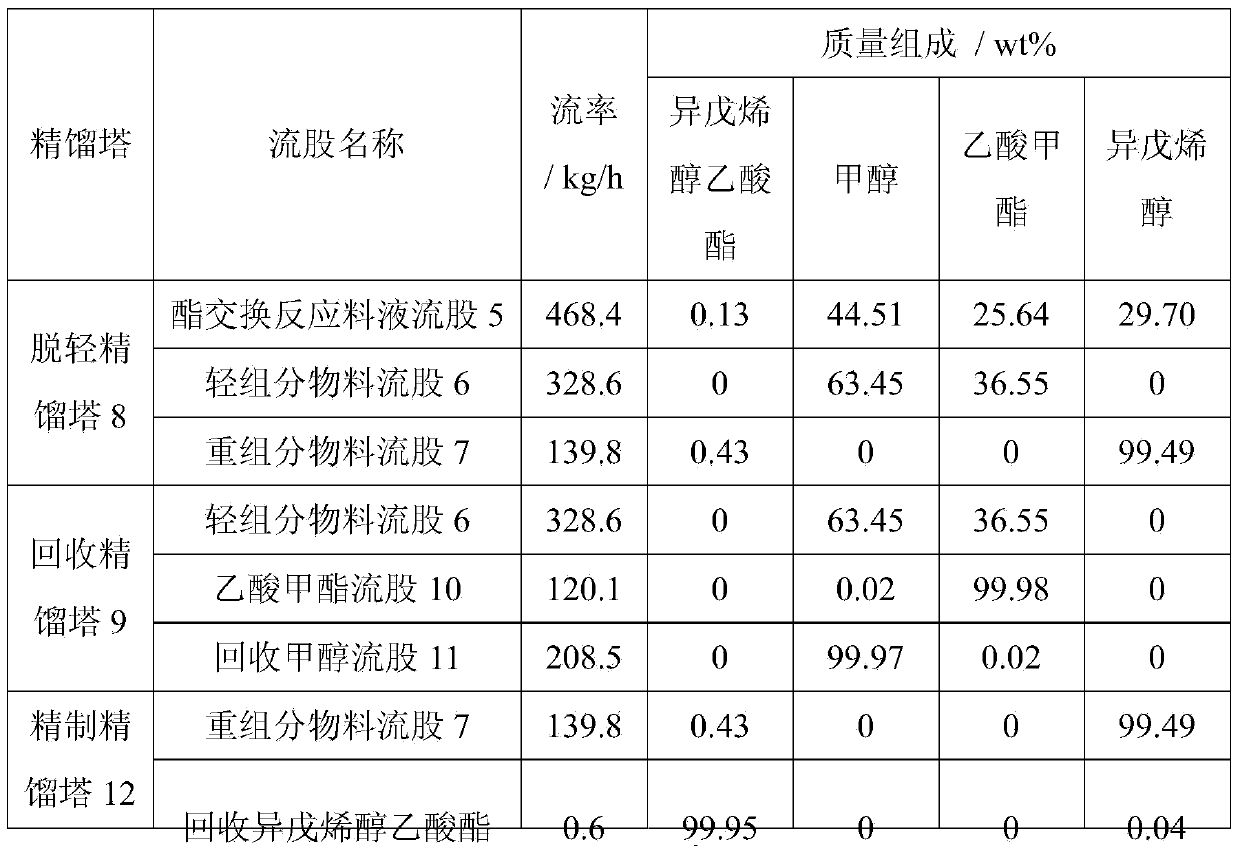
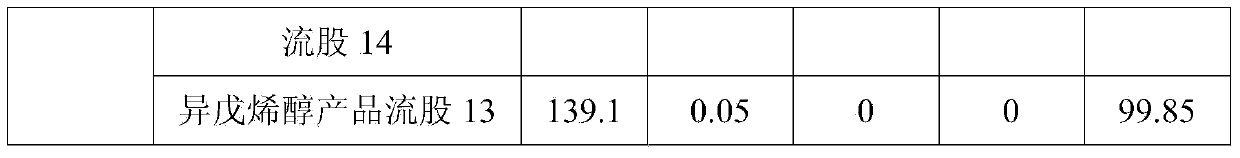
[0029] at 3m 3 In the transesterification reactor, add prenyl alcohol acetate 1000kg, methanol 1250kg, strong basic anion exchange resin 50kg, react at 50 ℃ for 3h, the conversion ratio of prenyl alcohol acetate after the reaction is 99.7 %, the yield of isopentenol is 99.4%. The obtained transesterification reaction liquid enters the continuous separation and purification process, and the mass fraction of the final product isopentenol can reach 99.85%. The flow rate of each stream is shown in Table 1.
[0030] Table 1
[0031]
[0032]
Embodiment 2
[0034] 5kg KOH solid was dissolved in 100L deionized water, 100kg SiO 2The solution was added to the above solution, and after stirring for 1 h, the water in the system was removed by a rotary evaporator. The obtained solid was vacuum dried at 80° C. for 24 h to obtain a solid base catalyst with a KOH loading of 5 wt %.
[0035] at 3m 3 In the transesterification reactor, add prenol acetate 1600kg, recovered prenol acetate 200kg, methanol 250kg, reclaimed methanol 200kg, KOH / SiO 2 18kg of solid base catalyst was reacted at 80° C. for 1 hour. After the reaction, the conversion rate of prenol acetate was 70.5%, and the yield of prenol was 70.3%. The obtained transesterification reaction solution enters the continuous separation and purification process, and the mass fraction of the final product isoprenol can reach 99.51%. The flow rates of each stream are shown in Table 2.
[0036] Table 2
[0037]
Embodiment 3
[0039] Dissolve 30kg of NaOH solid in 100L of deionized water, add 100kg of MCM-41 molecular sieves to the above solution, and after stirring for 1 hour, remove the water in the system by a rotary evaporator. The obtained solid was vacuum dried at 80° C. for 24 h to obtain a solid base catalyst with a NaOH loading of 30 wt %.
[0040] at 3m 3 In the transesterification reactor, add prenol acetate 1100kg, reclaimed prenol acetate 100kg, methanol 850kg, reclaim methanol 50kg, NaOH / MCM-41 solid base catalyst 36kg, react at 60 ℃ 2h, the conversion rate of prenol acetate was 85% after the reaction, and the yield of prenol was 84.6%. The obtained transesterification reaction solution enters the continuous separation and purification process, and the mass fraction of the final product isoprenol can reach 99.77%. The flow rates of each stream are shown in Table 3.
[0041] table 3
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com