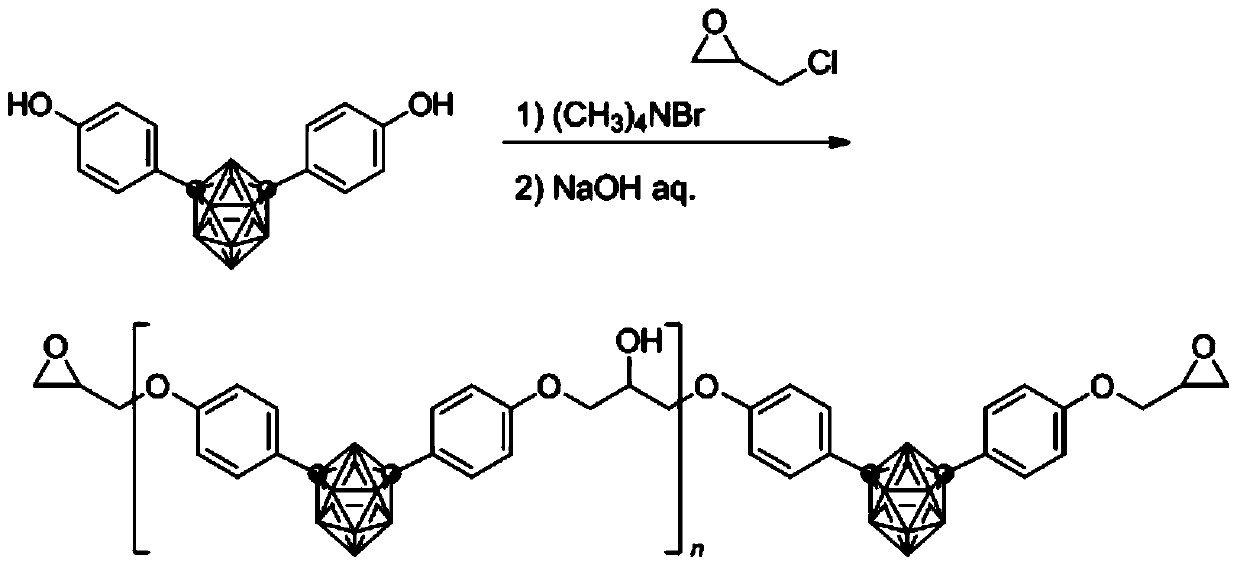
Synthesizing and curing method of carborane epoxy resin
A technology of epoxy resin and carborane, applied in epoxy resin glue, adhesive type, adhesive and other directions, can solve the problem that the addition reaction cannot be carried out
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
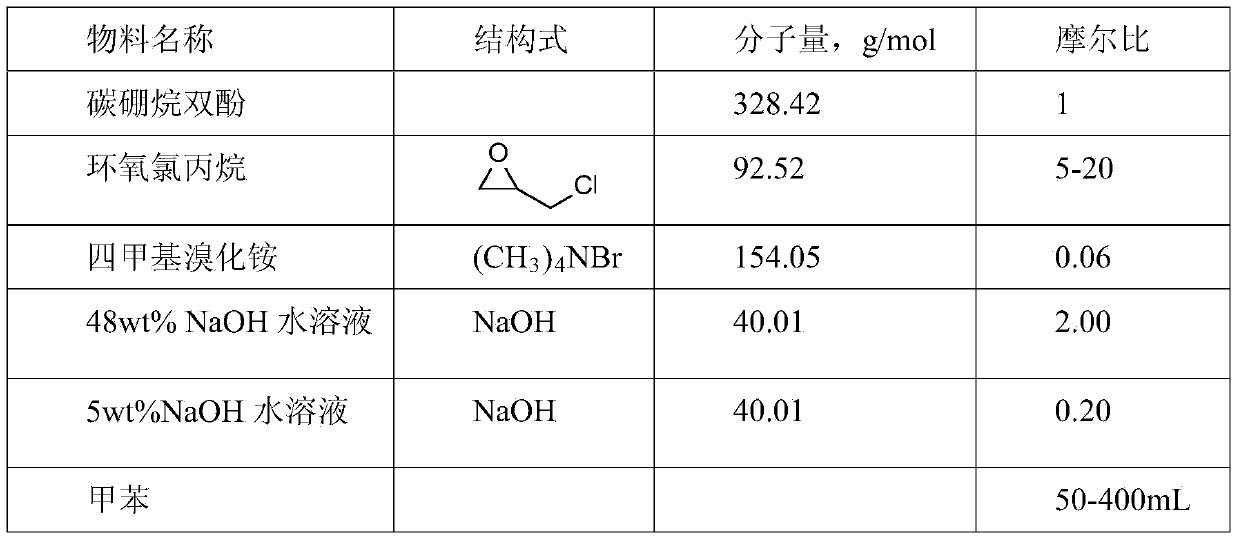
[0029] 1,7-carborane diphenol (0.5996 g), tetramethylammonium bromide (0.0169 g) and epichlorohydrin (1.6891 g) were added to a 100 mL two-necked flask. Install a condensing reflux system, evacuate nitrogen, and shake the flask during the heating process to completely dissolve 1,7-carborane diphenol and tetramethylammonium bromide in epichlorohydrin. Under the protection of nitrogen, the temperature was raised to 100 °C for 2 hours, and a light yellow solid appeared at the bottom of the flask; after the temperature was lowered to 85 °C, 48 wt% NaOH aqueous solution (0.15 g NaOH) was added dropwise with a needle tube within 1 hour, during which time azeotropic desorption under normal pressure Remove water, and then keep the temperature and heat for 1 hour to obtain a light yellow viscous liquid, and there is a light yellow solid insoluble matter at the bottom of the flask; remove the viscous liquid, remove epichlorohydrin by rotary evaporation, and add to the crude product Tolu...
Embodiment 2
[0031] 1,7-Carborane diphenol (0.6004 g), tetramethylammonium bromide (0.0170 g) and epichlorohydrin (1.2580 g) were added to a 100 mL two-necked flask. Install a condensing reflux system, evacuate nitrogen, and shake the flask during the heating process to completely dissolve 1,7-carborane diphenol and tetramethylammonium bromide in epichlorohydrin. Under the protection of nitrogen, the temperature was raised to 50°C for 6 hours, and a light yellow solid appeared at the bottom of the flask; after the temperature was lowered to 70°C, a 48wt% NaOH aqueous solution (0.15gNaOH) was added dropwise with a needle within 1 hour, during which the azeotropic removal of water, and then keep the temperature and heat for 1h to obtain a light yellow viscous liquid, and there is a light yellow solid insoluble matter at the bottom of the flask; remove the viscous liquid, remove epichlorohydrin by rotary evaporation, and add toluene to the crude product React with 5wt% NaOH aqueous solution a...
Embodiment 3
[0033] 1,2-Carborane diphenol (1.00 g), tetramethylammonium bromide (0.028 g) and epichlorohydrin (2.82 g) were added to a 100 mL two-necked flask. Install a condensing reflux system, evacuate nitrogen, and shake the flask during the heating process to completely dissolve 1,2-carborane diphenol and tetramethylammonium bromide in epichlorohydrin. Under the protection of nitrogen, the temperature was raised to 100 ° C for 2 hours, and a light yellow solid appeared at the bottom of the flask; after the temperature was lowered to 85 ° C, 48 wt% NaOH aqueous solution (0.26 g NaOH) was added dropwise with a needle tube within 1 hour, during which time azeotropic desorption under normal pressure Remove water, and then keep the temperature and heat for 1 hour to obtain a light yellow viscous liquid, and there is a light yellow solid insoluble matter at the bottom of the flask; remove the viscous liquid, remove epichlorohydrin by rotary evaporation, and add to the crude product Toluene...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| shear strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com