Preparation method of lithium ion battery positive pole active material
A positive electrode active material, lithium-ion battery technology, applied in the direction of battery electrodes, secondary batteries, fluoride preparation, etc., can solve the problems of poor conductivity of amorphous carbon layer, poor electrochemical performance of metal fluoride, etc., to achieve simple process, Effects of stable cycle performance and high Coulombic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
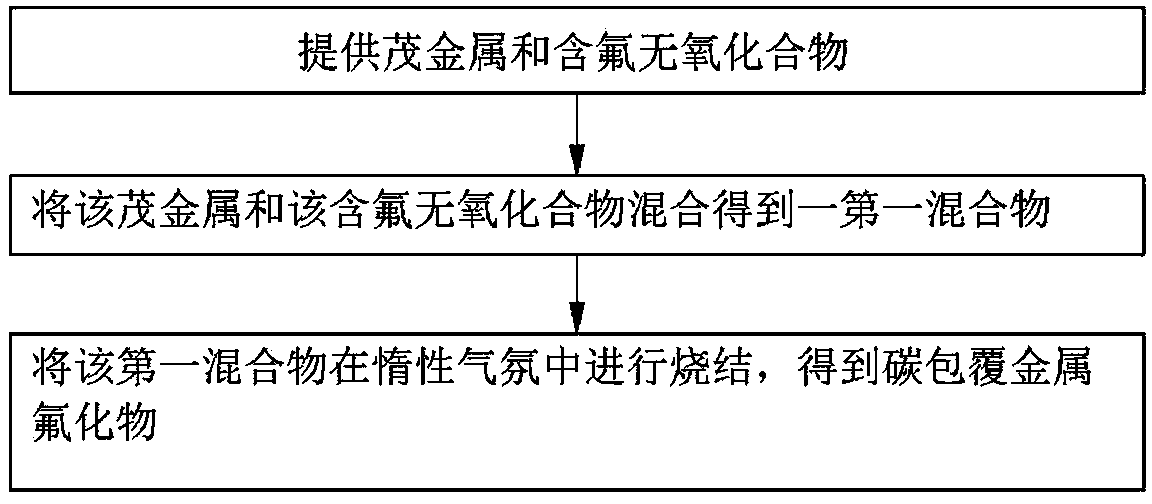
[0020] see figure 1 , the first embodiment of the present invention provides a method for preparing a metal fluoride positive electrode active material, comprising:
[0021] S11, providing metallocenes and fluorine-containing oxygen-free compounds;
[0022] S12, mixing the metallocene and the fluorine-containing oxygen-free compound to obtain a first mixture; and
[0023] S13, sintering the first mixture in an inert atmosphere to obtain a carbon-coated metal fluoride.
[0024] In step S11, the metallocene is an organometallic compound formed by linking a transition metal and cyclopentadiene. A typical metallocene is formed by linking two cyclopentadienyl anions and a metal center in a divalent oxidation state, the general formula is (C 5 h 5 ) 2 M. The metallocene can be decomposed into metal elements and carbon clusters during the sintering process. The carbon cluster refers to an atomic group composed of ten to hundreds of carbon atoms, and the carbon cluster has rela...
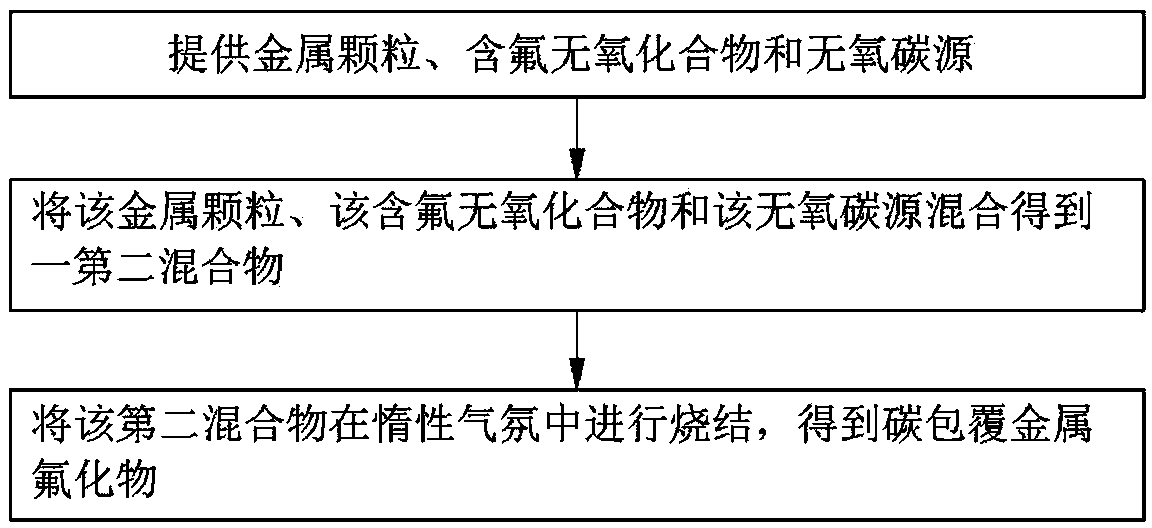
Embodiment 1
[0081] Ferrocene and PVDF were mixed according to the fluorine / iron element molar ratio of 2:1, and ball milled at a speed of 500 rpm / min for 2 hours to obtain a first mixture. The first mixture was placed in a stainless steel container, and the container was placed in a glove box filled with argon, and then the first mixture was reacted at 600° C. for 5 hours to obtain carbon-coated ferrous fluoride.
[0082] see Figure 5 , the carbon-coated ferrous fluoride is rod-shaped particles, the length of the rod-shaped particles is about 1 μm, the width is between 100 nm and 1 μm, and the thickness of the carbon layer is about 20 nm. see Figure 6, the diffraction peak of the XRD spectrum of the reaction product is consistent with the diffraction peak of the standard spectrum of ferrous fluoride, which proves that the above preparation method can prepare ferrous fluoride with pure phase and good crystallinity. see Figure 7 , the carbon layer of carbon-coated ferrous fluoride has...
Embodiment 2
[0084] Ferrocene and NH 4 F was mixed according to the fluorine / iron element ratio of 2.05:1, and ball milled at a speed of 400 rpm / min for 1 hour to obtain a first mixture. The first mixture was placed in a stainless steel container, the container was placed in a glove box and filled with nitrogen, and then the first mixture was reacted at 650° C. for an hour to obtain carbon-coated ferrous fluoride.
[0085] see Figure 8 , the carbon-coated ferrous fluoride is spherical particles, the diameter of the spherical particles is between 100nm and 1 μm, and the thickness of the carbon layer is about 10nm. When this carbon-coated ferrous fluoride is used for the positive electrode of lithium ion battery, it has 330mAh·g -1 The first lithium storage capacity, the coulombic efficiency is above 95%, and the capacity loss rate of each cycle within 40 cycles is 0.72%, please refer to Figure 9 , compared with the uncoated ferrous fluoride, the carbon-coated ferrous fluoride has bette...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com