A kind of chalcone derivative and its application
A technology of chalcone derivatives and drugs, which is applied in the field of chemical medicine, can solve the problem of low anti-free radical activity, and achieve the effect of strong ability to scavenge free radicals and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
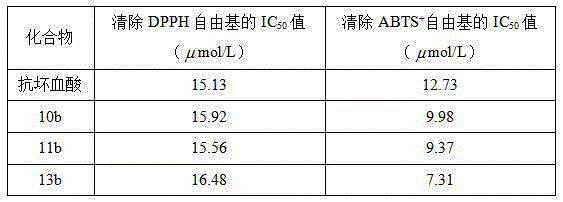
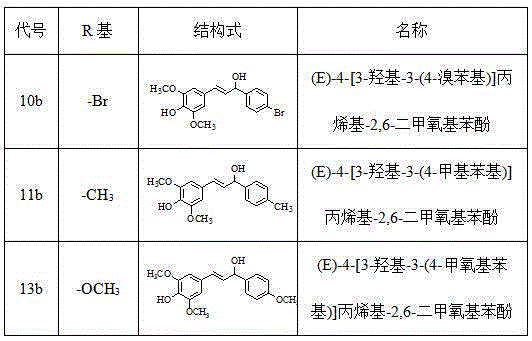
[0026] Example 1 Preparation of (E)-4-[3-hydroxyl-3-(4-bromophenyl)]propenyl-2,6-dimethoxyphenol (10b)
[0027]
[0028] 10b
[0029] Add 1.82g of syringaldehyde, 1.99g of 4-bromoacetophenone and 1mL of piperidine into a 50mL pear-shaped bottle, quickly place it in an oil bath at 160°C, and reflux for 1h while stirring to obtain a dark red viscous liquid. The progress of the reaction was followed by TLC. After cooling to room temperature, under ice bath and strong stirring, add very concentrated NaOH solution to adjust the pH to above 12. Add concentrated HCl to acidify to pH 1-2. Then add a large amount of distilled water and stir vigorously to obtain a yellow turbid liquid and an orange-red oily substance. Stirring was continued, the orange-red color gradually changed to yellow, and after standing still, a large amount of yellow solids were formed. Then keep it at low temperature for a long time until the orange-red color turns yellow, filter, add an appropriate amou...
Embodiment 2
[0031] Example 2 Preparation of (E)-4-[3-hydroxyl-3-(4-methylphenyl)]propenyl-2,6-dimethoxyphenol (11b)
[0032]
[0033] 11b
[0034] Add 1.82g of syringaldehyde, 1.34g of 4-methylacetophenone and 1mL of piperidine into a 50mL pear-shaped bottle, quickly place it in an oil bath at 160°C, and reflux for 1 hour while stirring to obtain a dark red viscous liquid. Add 2-3 mL of absolute ethanol and distilled water respectively, and continue the reaction for 20 min. The progress of the reaction was followed by TLC. After the reaction is cooled to room temperature, pour it into a beaker, add another 10-15mL of distilled water, stir well, add an appropriate amount of concentrated hydrochloric acid to the reaction solution under an ice bath, stir while adding, adjust the pH to 1-2, and add a large amount of distilled water , to obtain yellow turbid liquid and orange-red oil, continue to stir, orange-red gradually turns yellow, continue to stir in the ice bath, a large amount of...
Embodiment 3
[0036] Example 3 Preparation of (E)-4-[3-hydroxyl-3-(4-methoxyphenyl)]propenyl-2,6-dimethoxyphenol (13b)
[0037]
[0038] 13b
[0039] Add 1.82g of syringaldehyde, 1.50g of 4-methoxyacetophenone and 1mL of piperidine into a 50mL pear-shaped bottle, quickly place it in an oil bath at 160°C, and reflux for 1 hour while stirring to obtain a dark red viscous liquid. Add 2-3 mL of absolute ethanol and continue the reaction for 20 min. The progress of the reaction was followed by TLC. After the reaction, cool to room temperature, pour the reaction solution into a large amount of cold dilute hydrochloric acid solution under the conditions of ice bath and strong stirring, and then add a large amount of distilled water to obtain a yellow turbid liquid and an orange-red oil. Continue to stir, the orange-red color gradually turns yellow. Stirring was continued in the ice bath, and a large amount of khaki solid was formed. Stir until all the orange-red turns to khaki, and filter....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com