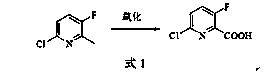6-chlorine-3-fluorine-2-picolinic acid synthesis process
A technology of picolinic acid and picoline, which is applied in the field of synthesis of 6-chloro-3-fluoro-2-picolinic acid, can solve the problems of no similar synthesis method, etc., achieve good economic and social benefits, and good reaction selectivity , the effect of less process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
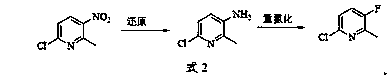
[0026] Embodiment 1: the synthesis of 6-chloro-3-amino-2-picoline
[0027] Add 300 ml of ethanol, 86.3 g (0.5 mol) of 6-chloro-3-nitro-2-methylpyridine, and 8.6 g of 5% palladium carbon catalyst into a 1-liter high-pressure hydrogenation reactor, start stirring, and heat to 40 ~45°C, feed high-purity hydrogen, maintain the hydrogen pressure of 0.3-0.5MPa, react for 6-8 hours, control the sampling and analysis of the raw material 6-chloro-3-amino-2-picoline content below 0.3%, stop Reaction, lowered to room temperature, filtered out the catalyst, obtained filtrate, evaporated ethanol under reduced pressure to obtain a gray solid, recrystallized with a mixed solution of ethyl acetate and cyclohexane, dried to obtain 56.1g of the product, and the relative content of the liquid phase was more than 98% , the solid can be directly used in the further reaction of Example 2 below.
Embodiment 2
[0028] Example 2: Synthesis of 6-chloro-3-fluoro-2-picoline
[0029] Add 460 milliliters of 20% hydrochloric acid in a 2000 milliliter three-necked bottle equipped with mechanical stirring, then add 71.3 g (0.5 mol) of 6-chloro-3-amino-2-picoline prepared according to the method of Example 1, fully Stir, use an ice-salt bath to control the temperature of the reaction system at 0-3°C, slowly add a sodium nitrite aqueous solution (containing 38.0 g of sodium nitrite and 38.0 ml of water) dropwise, and the dropwise addition takes 1 hour. After the dropwise addition, maintain the Stir at temperature for 0.5 hour, add dropwise 270 ml of 48% fluoroboric acid aqueous solution, the temperature during the dropwise addition does not exceed 5°C, after the dropwise addition, slowly rise to room temperature for 2 hours of reaction. Pour the reaction solution into ice water, stir well, neutralize it with 10% sodium hydroxide aqueous solution to pH = 7, add the extractant chloroform, extract...
Embodiment 3
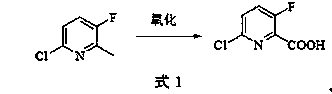
[0030] Example 3: Synthesis of 6-chloro-3-fluoro-2-pyridinecarboxylic acid
[0031] In the reaction flask, add 400ml of 40% dilute sulfuric acid, 176g of potassium dichromate, sodium tungstate dihydrate (Na 2 WO 4 2H 2 O) 4.4g, phase transfer catalyst (crown ether 18 crown 6) 0.8g, 6-chloro-3-fluoro-2-methylpyridine 43.6g (0.3mol) prepared according to the method of Example 2, heated to 105°C and react for 6 hours, pour the reaction solution into 3kg of crushed ice, filter with suction, wash the filter cake with an appropriate amount of ice water, heat and dissolve the filter cake in aqueous potassium carbonate solution, and extract unreacted raw materials with chloroform after cooling and other impurities, the water layer was acidified with 20% hydrochloric acid, cooled, the precipitated solid was suction filtered, and dried to obtain 48.5 g of 6-chloro-3-fluoro-2-pyridinecarboxylic acid as a white solid, with a yield of 92.0% and a liquid phase content of 98.7 %. 6-Chlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com