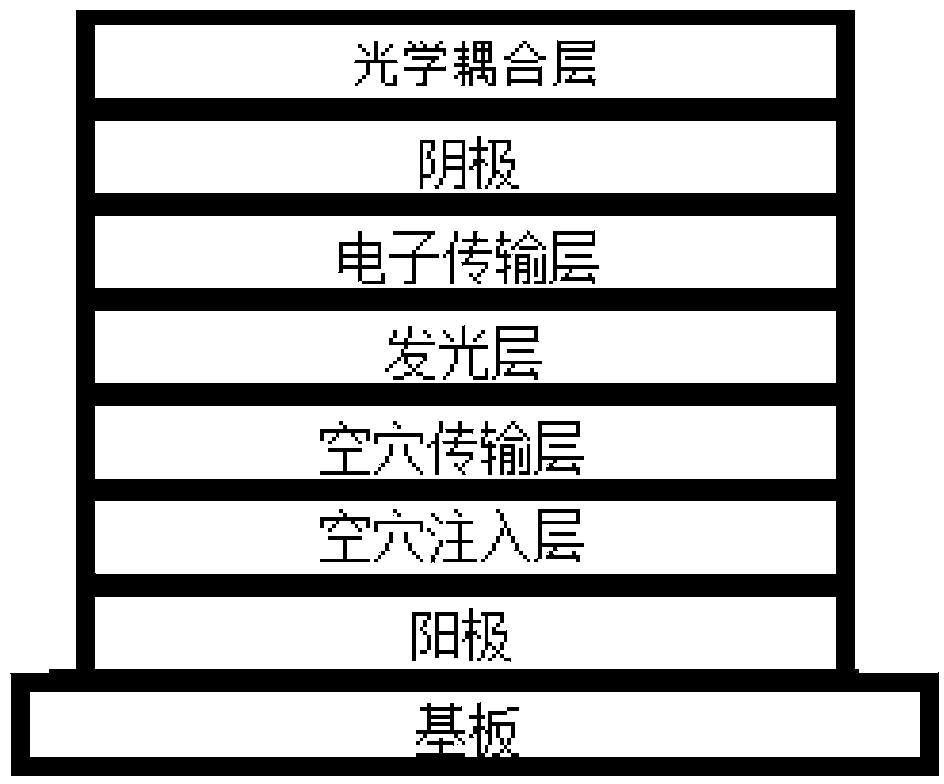
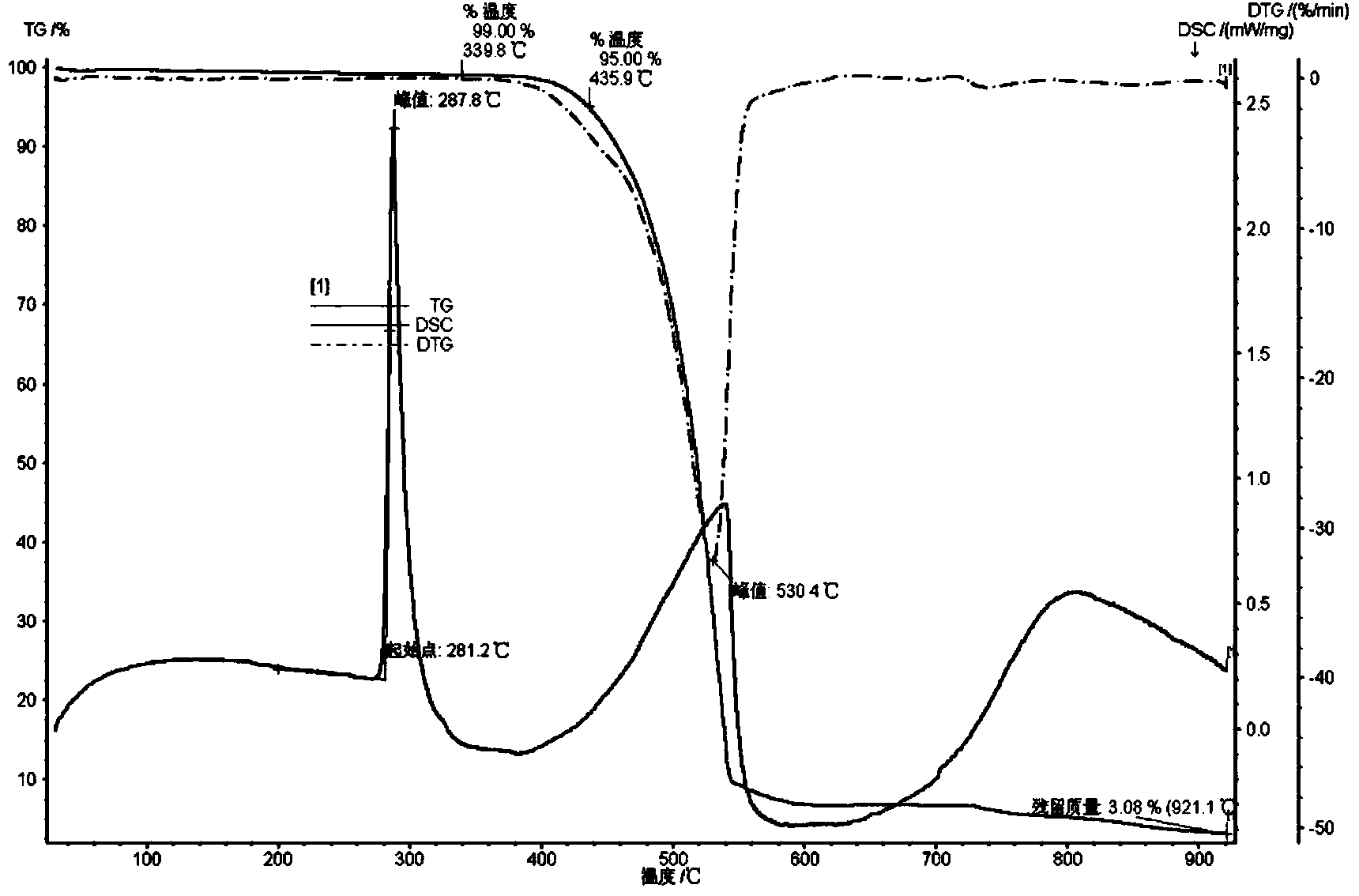
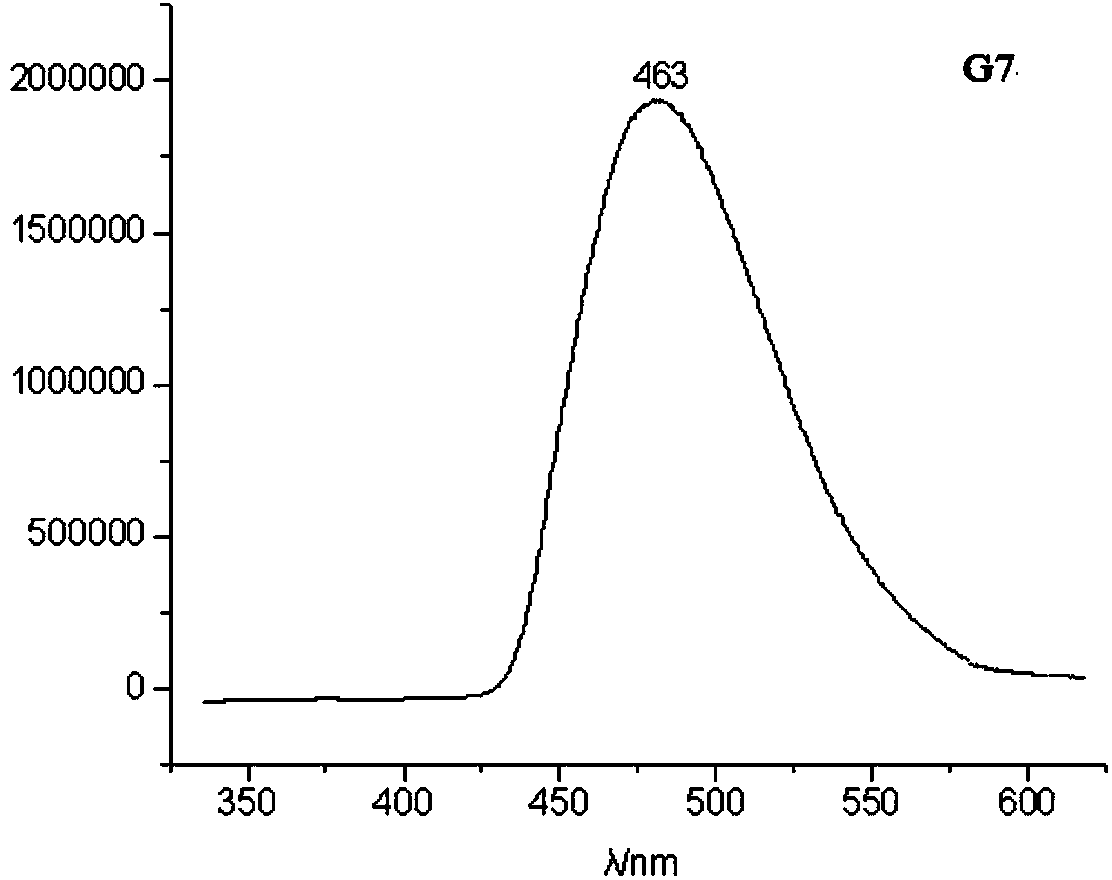
Organic compound and electroluminescent device using same
An electroluminescent device, an organic compound technology, applied in the field of organic nitrogen heterocyclic derivatives and its application in the field of electroluminescent display technology, can solve the problems of unsatisfactory luminous efficiency, lifespan and optical purity , to achieve high carrier injection and transport capabilities, high brightness, and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] 1. The preparation of core compound A, its structural formula and synthetic route are as follows:
[0033]
[0034] (1) Synthesis of intermediate A-1:
[0035] At -78°C, add 12mL of n-BuLi (2.5mole / L, 0.03mole) dropwise to 6.16g (0.02mole) 3,5-diphenylbromobenzene and 150ml THF and keep warm for 30 minutes, then at -78°C 3.32 ml of trimethyl borate (0.03 mole) was added dropwise, and reacted for 2 hours after the addition was completed, to terminate the reaction. Then, 50 ml of water was added to the obtained reaction solution, stirred for 20 minutes, adjusted to acidity by adding hydrochloric acid, and stirred for 30 minutes. Then the above-mentioned reaction solution was extracted three times with ethyl acetate, the organic phases were combined and washed with water, the organic phase was separated, the organic liquid was spin-dried, and the obtained solid was boiled with petroleum ether to obtain 5.48 g of off-white solid. Body A-1, yield 50%.
[0036] (2) Synt...
Embodiment 1
[0078] Example 1 Preparation of Compound G1
[0079] The compound G1 to be prepared in this example has a structural formula and a synthetic route as follows:
[0080]
[0081] Preparation of Compound G1:
[0082] Mix 5.5g (0.01mole) of core compound A, 2.87g (0.01mole) of 9-phenylcarbazole-3-boronic acid, 4.15g (0.03mole) of potassium carbonate, 50ml of toluene, 30ml of ethanol and 30ml of water. 0.23 g (0.0002 mole) of tetrakis(triphenylphosphine) palladium was added, the temperature was raised to reflux overnight, and the reaction was stopped until the plate was monitored until the reaction was complete. The obtained reaction solution is spin-dried, and the solid obtained by spin-drying is added to 100ml of dichloromethane, passed through a silica gel column, the filtrate is added with 100ml of water, washed with water and separated to obtain an organic phase, and the organic phase is spin-dried and boiled with ethanol to obtain 5.2 g of white solid compound G1, yield ...
Embodiment 2
[0083] Example 2 Preparation of Compound G2
[0084] The compound G2 to be prepared in this example has a structural formula and a synthetic route as follows:
[0085]
[0086] Preparation of Intermediate G2-1:
[0087] Mix 1.69g (0.01mole) of 4-aminobiphenyl, 2.73g (0.01mole) of 2-bromo-9,9-dimethylfluorene and 50ml of toluene, and add 0.18g (0.0002mole) of Pd under nitrogen atmosphere 2 (dba) 3 , 1.68g (0.015mole) potassium tert-butoxide and 0.809g (0.0004mole, 10% toluene solution) tri-tert-butylphosphine, heated to 70 ° C, spot plate monitoring, after the reaction is complete, cool to room temperature, and then in the obtained reaction 100ml of toluene was added to the compound, and the filtrate was obtained through a silica gel funnel, and the filtrate was extracted with water to obtain an organic phase. After the organic phase was spin-dried, the solid obtained by spin-drying was recrystallized with an ethanol-toluene system to obtain 2.7 g of solid intermediate G2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com