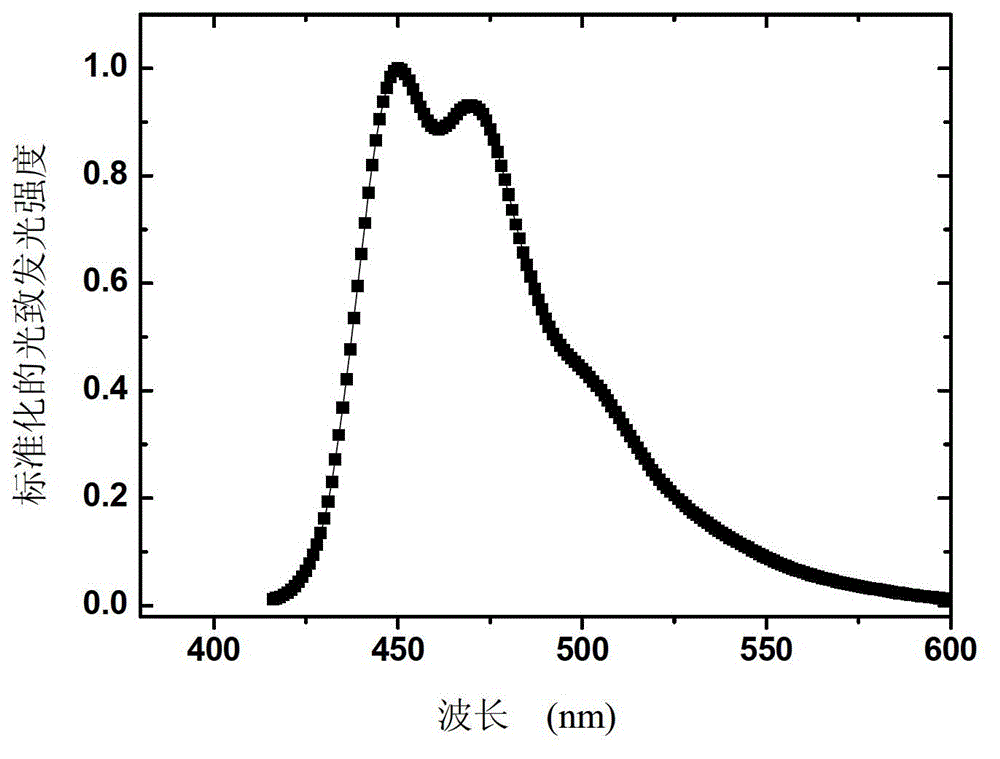
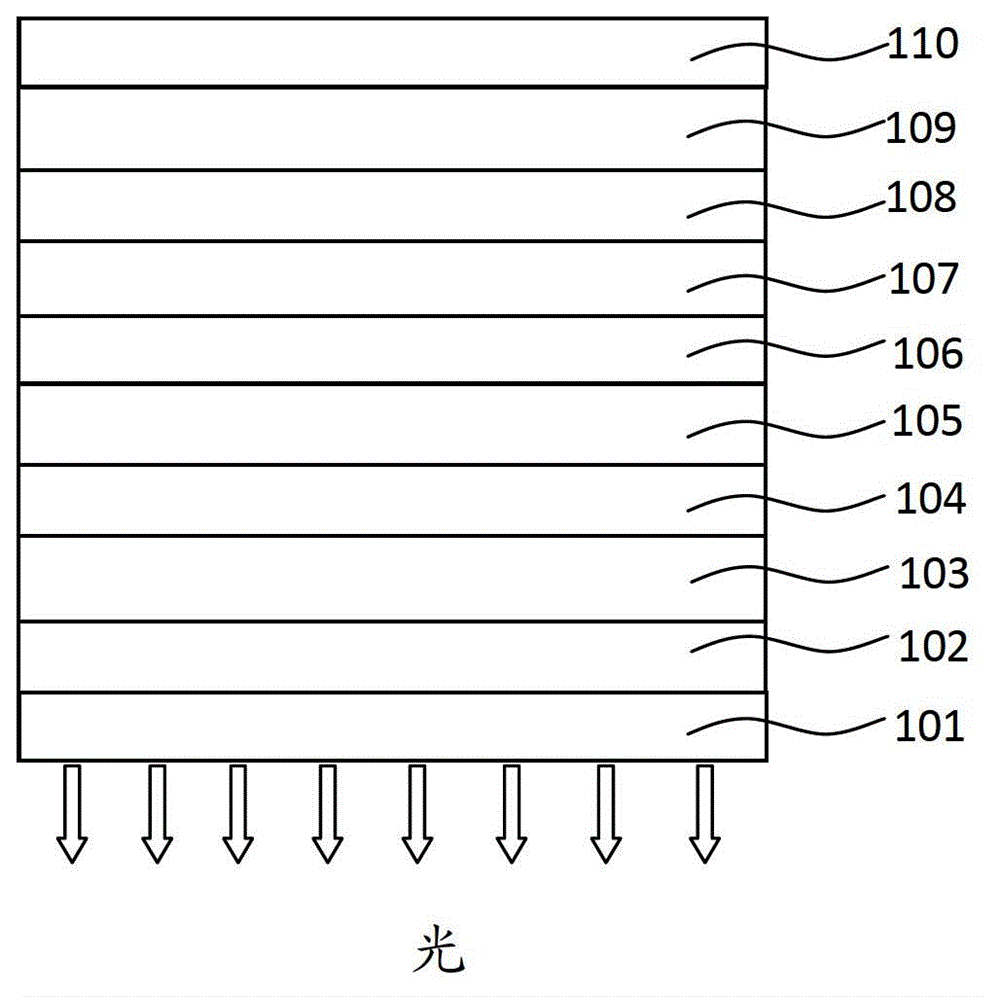
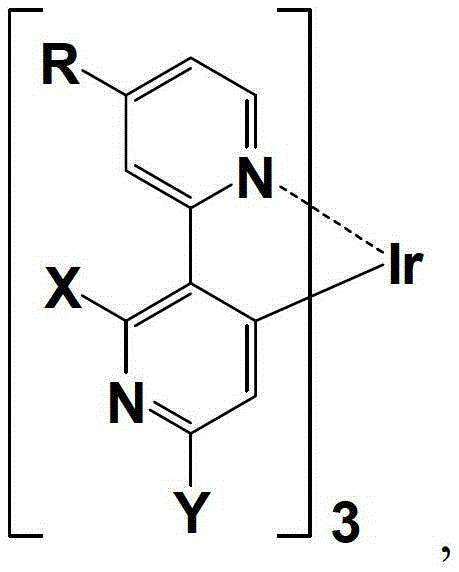
Organic phosphorescence material and preparation method thereof and organic electroluminescent device
A technology of optical materials and organophosphorus, applied in luminescent materials, electrical solid devices, organic chemistry, etc., can solve the problems of luminous color purity, luminous efficiency, insufficient attenuation of device efficiency, poor stability, short life, etc., and achieve processing costs Inexpensive, easy to control the preparation process, and good luminescent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]Example 1: An organic phosphorescent material complex tris(2',6'-dichloro-4-amino-2,3'-bipyridine-N,C2') iridium, as shown in the following structural formula:
[0049]
[0050] The preparation method of the above-mentioned organic phosphorescent material comprises the following steps:
[0051] (1) Provide compound C1 and compound D1 represented by the following structural formula respectively:
[0052]
[0053] (2) Synthesis of 2,6-dichloro-4'-amino-3,2'-bipyridine
[0054]
[0055] Under nitrogen protection, (1.04g, 6.00mmol) 4-amino-2-bromopyridine, (1.38g, 7.20mmol) 2,6-dichloro-3-pyridineboronic acid, (0.35g, 0.30mmol) Pd(PPh 3 ) 4 After dissolving in 30mL toluene, add 22mmol mass fraction of 5% K 2 CO 3 Aqueous solution, heated to reflux, stirred for 24h. After cooling to room temperature, distilled water was added and extracted three times with 100 mL of ethyl acetate. The organic phases were combined and dried over anhydrous magnesium sulfate. Filt...
Embodiment 2
[0070] Example 2: An organic phosphorescent material complex tris(2'-fluoro-6'-chloro-4-amino-2,3'-bipyridine-N,C 2 ') iridium, as shown in the following structural formula:
[0071]
[0072] The preparation method of the above-mentioned organic phosphorescent material comprises the following steps:
[0073] (1) Provide compound C2 and compound D2 represented by the following structural formula respectively:
[0074]
[0075] (2) Synthesis of 2-fluoro-6-chloro-4'-amino-2',3-bipyridine
[0076]
[0077] Under nitrogen protection, (1.04g, 6.00mmol) 4-amino-2-bromopyridine, (1.58g, 9.00mmol) 2-fluoro-6-chloro-3-pyridineboronic acid, (0.07g, 0.06mmol) Pd( PPh 3 ) 4 After dissolving in 60mL toluene, add 12mmol mass fraction of 5% K 2 CO 3 Aqueous solution, heated to reflux, stirred for 24h. After cooling to room temperature, distilled water was added and extracted three times with 100 mL of ethyl acetate. The organic phases were combined and dried over anhydrous mag...
Embodiment 3
[0091] Example 3: An organic phosphorescent material complex tris(2'-chloro-6'-fluoro-4-amino-2,3'-bipyridine-N,C 2 ') iridium, as shown in the following structural formula:
[0092]
[0093] The preparation method of the above-mentioned organic phosphorescent material comprises the following steps:
[0094] (1) Provide compound C3 and compound D3 represented by the following structural formulas respectively:
[0095]
[0096] (2) Synthesis of 2-chloro-6-fluoro-4'-amino-2',3-bipyridine
[0097]
[0098] Under nitrogen protection, (1.04g, 6.00mmol) 4-amino-2-bromopyridine, (1.58g, 9.00mmol) 2-chloro-6-fluoro-3-pyridineboronic acid, (0.07g, 0.06mmol) Pd( After PPh3)4 was dissolved in 60mL DMF, 12mmol mass fraction of 5% K was added 2 CO 3 Aqueous solution, heated to reflux, stirred and reacted for 26h. After cooling to room temperature, distilled water was added and extracted three times with 100 mL of ethyl acetate. The organic phases were combined and dried over ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com