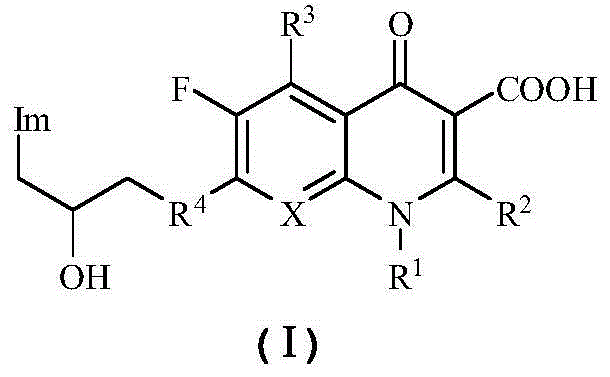
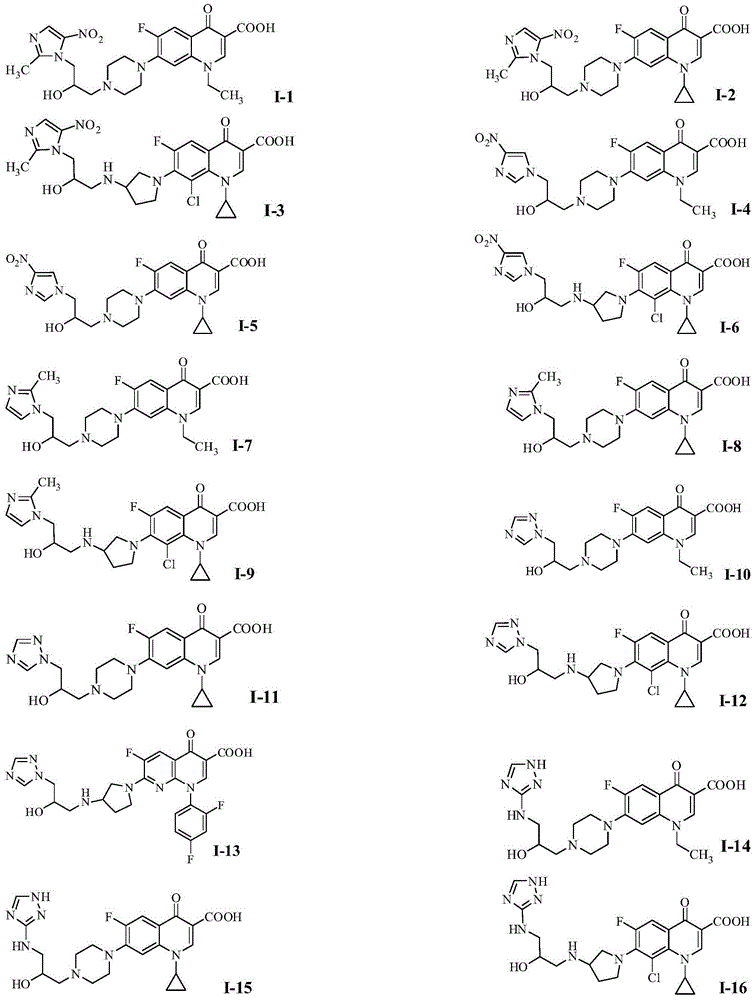
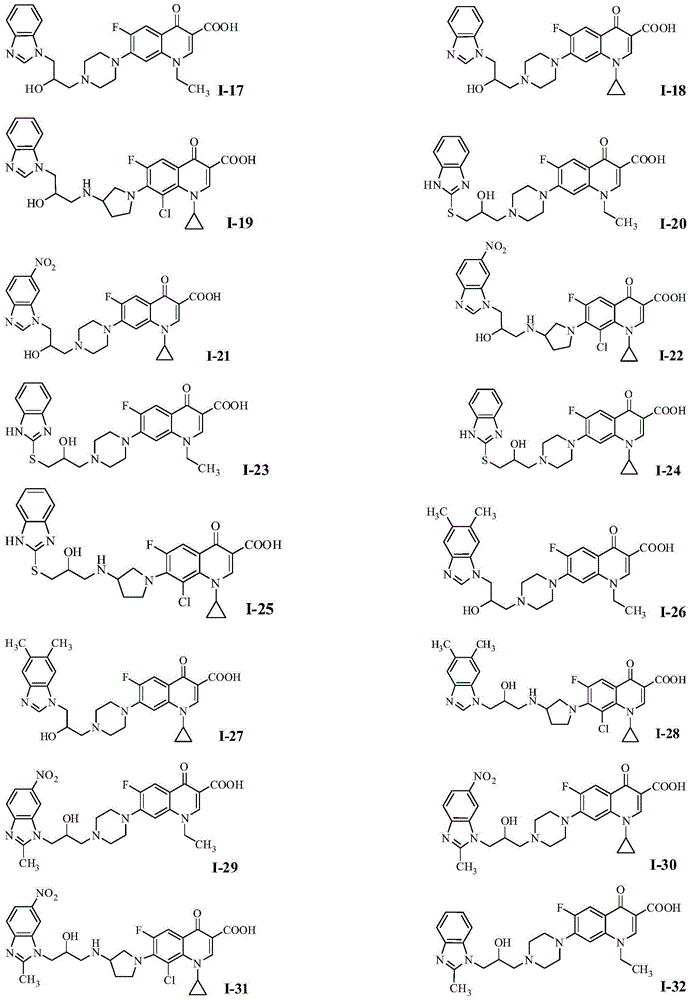
Quinolone azole alcohol compounds as well as preparation method and application thereof
A technology of quinolazolol and quinolones, which is applied in the field of chemistry, can solve the problems of serious bacterial drug resistance, etc., and achieve the effects of solving drug resistance, easy-to-obtain raw materials, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, the preparation of compound II-1
[0035]
[0036] In a 100mL round bottom flask, norfloxacin hydrochloride (0.96g, 0.0027mol), sodium bicarbonate (0.69g, 0.0048mol) and an appropriate amount of acetonitrile were stirred at a temperature of 50°C for 0.5h, cooled to room temperature, and added to the ring Oxychloropropane (1.00g, 0.0027mol) was kept under temperature control at 45°C and continued to stir, followed by thin-layer chromatography until the end of the reaction, and then concentrated, extracted, separated by column chromatography, recrystallized, and dried to obtain 0.68g of compound II-1. The yield was 67.4%.
[0037] Compound II-1: white powder; melting point 185-186°C; 1 H NMR (300MHz, CDCl 3 ):δ15.05(s,1H,COOH),8.63(s,1H,quinolone2-H),7.99(d,J=13.0Hz,1H,quinolone5-H),6.80(d,J=6.8Hz,1H ,quinolone8-H), 4.53(t, J=8.3Hz, 1H, CH 2 ), 4.30 (dd,2H,quinolone N-CH 2 ), 3.59(t, J=5.0Hz, 1H, O-CH), 3.32(t, J=14.3Hz, 4H, piperazine N-CH 2 ), 2.8...
Embodiment 2
[0038] Embodiment 2, the preparation of compound II-2
[0039]
[0040]In a 100mL round bottom flask, ciprofloxacin hydrochloride (0.99g, 0.0027mol), sodium bicarbonate (0.69g, 0.0048mol) and appropriate amount of acetonitrile were stirred at 50°C for 0.5h, cooled to room temperature, and added to Oxychloropropane (1.00g, 0.0027mol) was kept under temperature control at 45°C and continued to stir, followed by thin-layer chromatography until the end of the reaction, and then concentrated, extracted, separated by column chromatography, recrystallized, and dried to obtain 0.67g of compound II-2. The yield was 64.0%.
[0041] Compound II-2: yellow powder; melting point 200-202°C; 1 H NMR (600MHz, DMSO-d 6 ): δ15.19(s,1H,COOH),8.63(s,1H,quinolone2-H),7.85(dd,J=10.9,7.6Hz,1H,quinolone5-H),7.54(d,J=6.1Hz ,1H,quinolone8-H),5.13(d,J=4.5Hz,1H,quinolone N-CH),3.91(td,J=9.9,4.8Hz,1H,O-C-H),3.81(s,2H,O-CH 2 ),3.71-3.58(m,4H,piperazine N-CH 2 ), 2.67 (td, J=10.9, 6.3Hz, 4H, piperazi...
Embodiment 3
[0042] Embodiment 3, the preparation of compound II-3
[0043]
[0044] In a 100mL round bottom flask, Clinfloxacin hydrochloride (5.00g, 0.0137mol), sodium bicarbonate (4.60g, 0.0548mol) and an appropriate amount of ethanol were stirred at a temperature of 50°C for 0.5h, cooled to room temperature, and epoxy chloride was added. Propane (2.50g, 0.0274mol) was kept under temperature control at 45°C and continued stirring, tracked by thin-layer chromatography until the end of the reaction, and then concentrated, extracted, separated by column chromatography, recrystallized, and dried to obtain 3.61g of compound II-3, with a yield of 62.4% .
[0045] Compound II-3: yellow powder; melting point 191-192°C; 1 H NMR (600MHz, DMSO-d 6 ):δ14.56(s,1H,COOH),8.83(s,1H,quinolone2-H),7.93(d,J=11.9Hz,1H,quinolone5-H),4.42-4.37(m,1H,NH- CH 2 ),3.92-3.86(m,1H,NH-CH 2 ), 3.70(dd, J=11.0, 4.1Hz, 1H, cyclopropyl-CH), 3.60(dd, J=11.0, 5.5Hz, 1H, O-C-H), 3.34(s, 4H, pyrrole N-CH 2 ), 2.63 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com