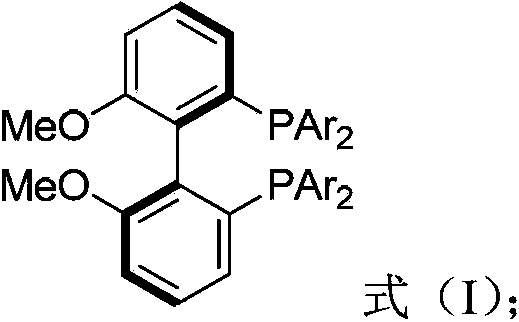
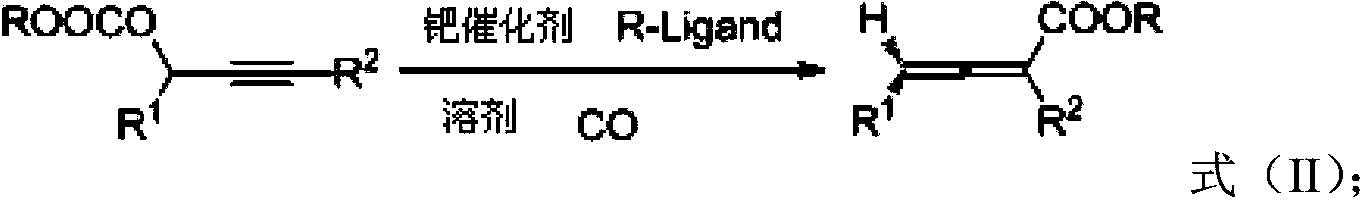Biphenyl ligand, synthetic method thereof and application thereof in methoxyl carbonylation reaction of racemized propargyl alcohol carbonate
A technology of propargyl carbonate and biphenyl ligands, which is applied in the direction of carbon monoxide or formate reaction preparation, asymmetric synthesis, organic chemical methods, etc., and can solve the problem that the response time is more than 48 hours and the universal range of substrates is narrow , harsh reaction conditions and other issues, to achieve the effect of increased enantioselectivity, faster reaction rate, and good reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
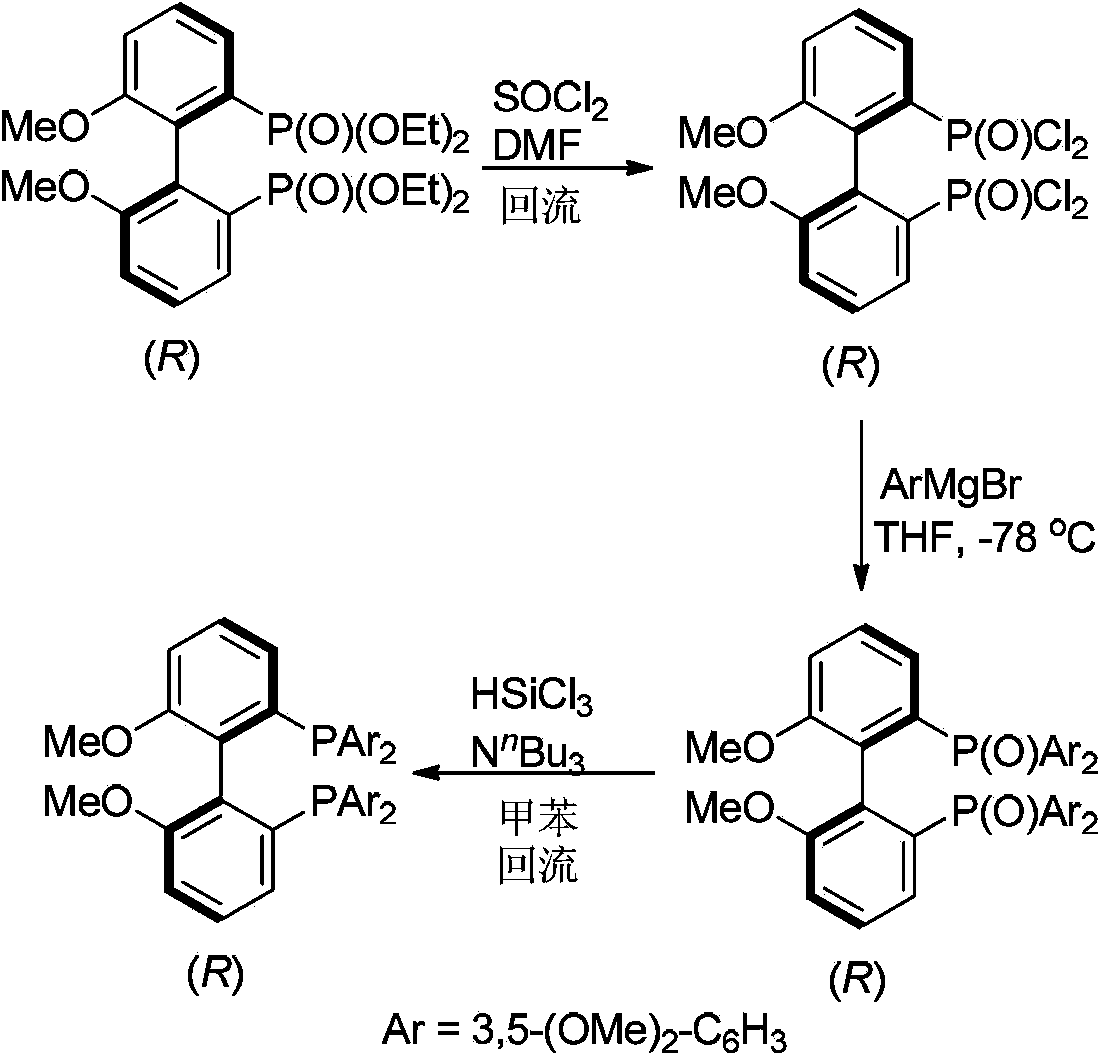
[0031] Example 1R-(+)-2,2'-bis(dichlorophosphinoxy)-6,6'-dimethoxy-1,1'-biphenyl (wyl-13-137)
[0032]
[0033] Add R-(+)-2,2'-bis(diethoxyphosphinoxy)-6,6'-dimethoxy-1,1'-biphenyl (2.4309g, 5mmol) in turn to the one-necked bottle , N,N'-dimethylformamide (0.5mL) and thionyl chloride (5mL), the single-necked bottle was placed in an oil bath at 90°C and heated to reflux for 9.8 hours. After the reaction system was cooled to room temperature, a large amount of thionyl chloride was pumped away with a water pump, and then the crude product was pumped dry with an oil pump to obtain 2.1731 g of a tan solid, with a crude yield of 97%. The resulting crude product was directly used in the next step.
[0034] 1 H NMR (300MHz, CDCl 3 )δ7.73-7.68 (m, 1H, ArH), 7.66-7.54 (m, 3H, ArH), 7.27-7.20 (m, 2H, ArH), 3.78 (s, 6H, 2x CH 3 ); 31 P NMR (121MHz, CDCl 3 )δ33.97.
Embodiment 2
[0035] Example 2R-(+)-2,2'bis(bis(3,5-dimethoxyphenyl)phosphineoxy)-6,6'-dimethoxy-1,1'-biphenyl ( wyl-13-139)
[0036]
[0037] Under the protection of argon, put magnesium chips (264.5 mg, 11 mmol) into a dry three-necked bottle, vacuumize and bake with a heat gun at the same time, and replace the bottle with argon atmosphere after cooling. Add freshly distilled tetrahydrofuran (15 mL) and a grain of iodine into the reaction flask, and stir at room temperature. 3,5-Dimethoxybromobenzene (2.1708g, 10mmol) was completely dissolved in freshly distilled tetrahydrofuran (5mL), and the solution was dropped into a three-neck flask within 15 minutes, stirred at room temperature for 2 hours, and set aside.
[0038] Under the protection of argon, add the R-(+)-2,2'bis(dichlorophosphinyloxy)-6,6'-dimethoxy-1,1 prepared in Example 1 into a dry three-neck flask '-biphenyl (448.2 mg, 1 mmol) and freshly distilled tetrahydrofuran (20 mL). Place the reaction bottle in a -78°C cold bat...
Embodiment 3
[0040] Example 3R-(+)-2,2'-bis(bis(3,5-dimethoxyphenyl)phosphine)-6,6'-dimethoxy-1,1'-biphenyl (wyl -13-143)
[0041]
[0042] Under argon protection, add the R-(+)-2,2' bis(bis(3,5-dimethoxyphenyl) Phosphinoxy)-6,6'-dimethoxy-1,1'-biphenyl (598.9 mg, 0.7 mmol) and freshly distilled toluene (20 mL). After stirring at room temperature, tri-n-butylamine (1.7 mL, d=0.778, 1.3226 g, 7 mmol) and trichlorosilane (0.7 mL, d=1.347, 942.9 mg, 7 mmol) were added successively. The reaction was heated to reflux at 130°C and stirred for 9 hours. Then the reaction was cooled to 0°C, and degassed 20% sodium hydroxide aqueous solution was slowly added to the reaction to quench the reaction (80mL), extracted with ethyl acetate (80mL x3), and the organic phases were combined and washed with water and saturated brine successively (80 mL each), dried over anhydrous sodium sulfate, filtered, concentrated, and 385.9 mg of a white solid product can be obtained by flash column chromatography, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com