Method for synthesizing alpha-pinene or beta-pinene by adopting biological process
A biological method, pinene technology, applied in the field of preparing isoprene derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
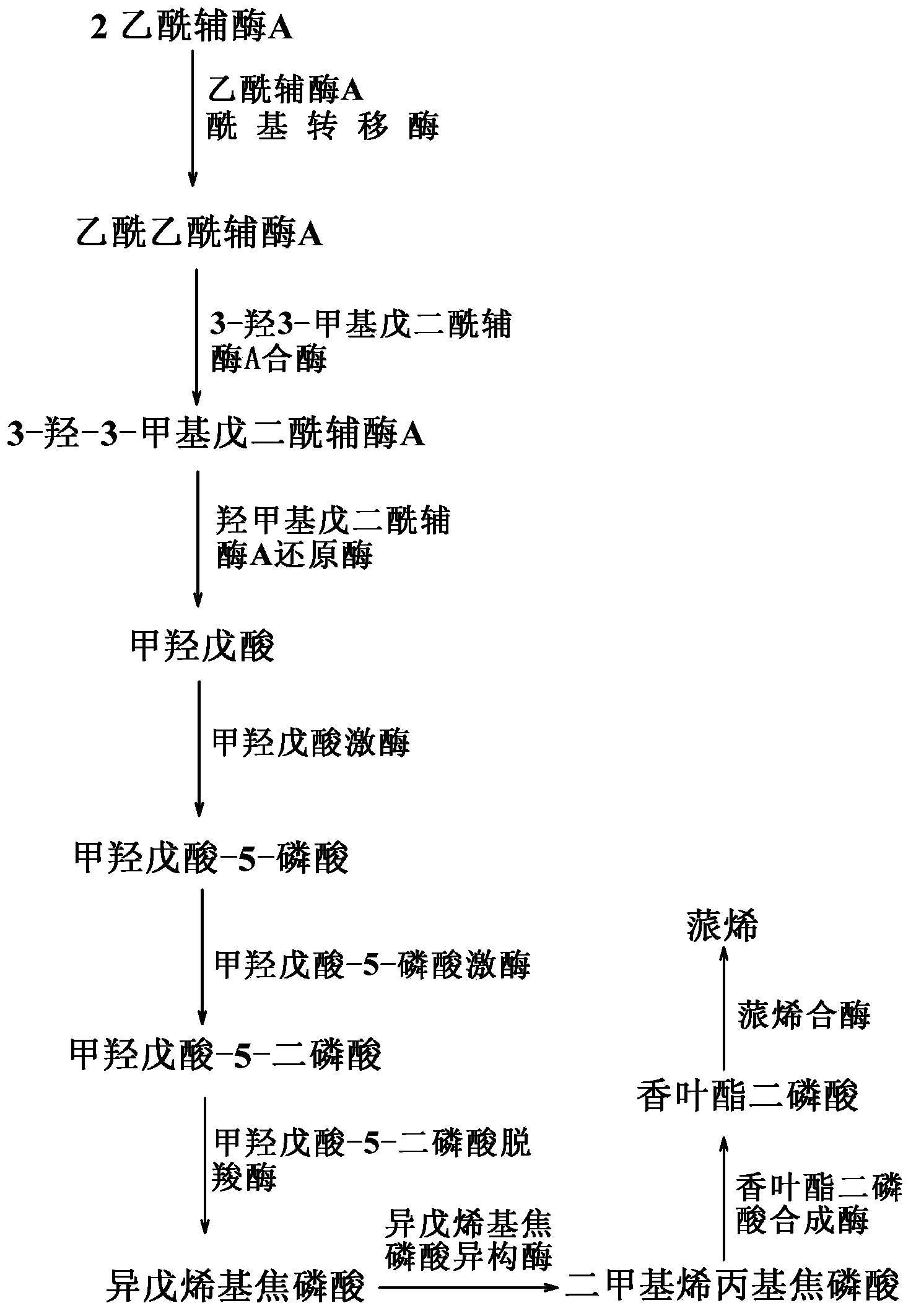
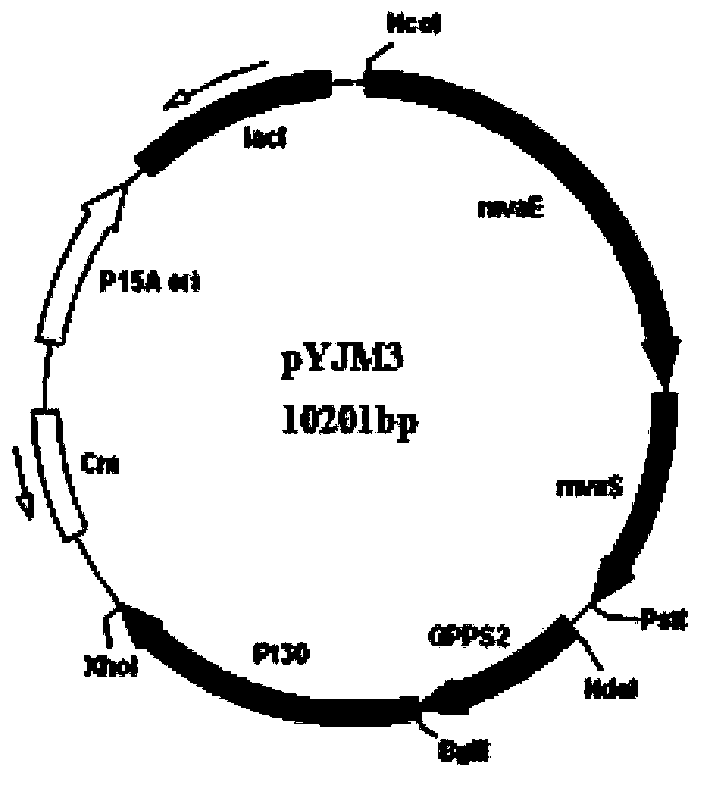
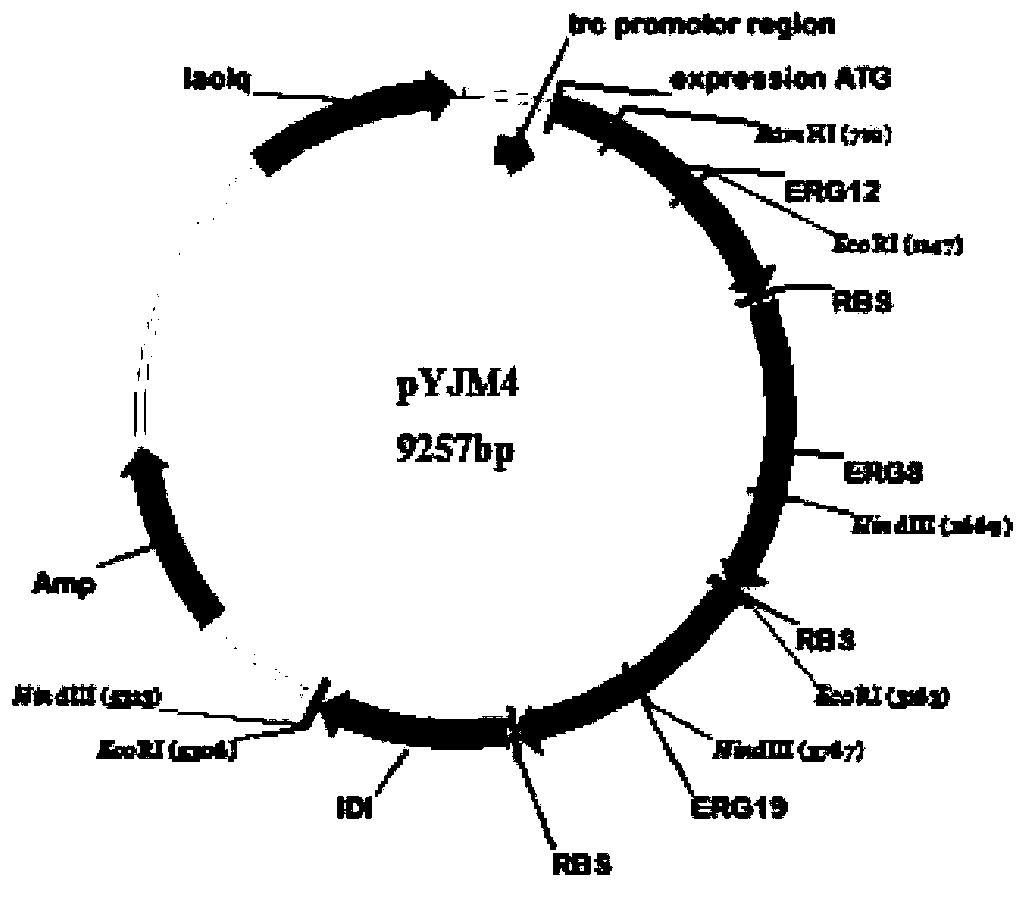
[0059] By co-expressing the acetyl-CoA acyltransferase gene / hydroxymethylglutaryl-CoA reductase gene (mvaE, SEQ ID NO: 1) from Enterococcus faecalis in Escherichia coli, 3-hydroxy- 3-methylglutaryl-CoA synthase gene (mvaS, SEQ ID NO: 2); mevalonate kinase gene (ERG12, SEQ ID NO: 5) derived from Saccharomyces cerevisiae, mevalonate-5- Phosphokinase gene (ERG8, SEQ ID NO:6), mevalonate-5-diphosphate decarboxylase gene (ERG19, SEQ ID NO:7), isopentenyl pyrophosphate isomerase gene (IDI1, SEQ ID NO : 8); the geranyl diphosphate synthase gene (GPPS2, SEQ ID NO: 3) derived from North American fir (Abiesgrandis) and the α-pinene synthase gene (Pt30, SEQ ID NO: 3) derived from loblolly pine (Pinustaeda) NO: 4), the biosynthesis of isoprene derivative - α-pinene, using the intermediate product of glucose degradation, acetyl-CoA.
[0060] 1.1 Cloning of foreign genes and construction of expression vectors
[0061] 1.1.1 Cloning of foreign genes
[0062] 1.1.1.1 Cloning of genes in the ...
Embodiment 2
[0086] The constructed plasmid was transformed into Escherichia coli competent cells, and the recombinant bacteria were fermented and cultured by shaking flask fermentation and fermenter fermentation, and the fermentation products were detected qualitatively and quantitatively by gas chromatography.
[0087] 2.1 Construction of E.coli recombinant strain
[0088] Transform E.coli BL21 (DE3) competent cells with pYJM27 (pACY-mvaE-mvaS-GPPS2-Pt30) and pYJM14 (pTrc-low) recombinant plasmids together by heat shock, and apply them to the antibiotics added with chloramphenicol and ampicillin The LB solid plate was obtained by PCR screening to obtain positive clones, thereby obtaining engineering Escherichia coli containing pYJM27 and pYJM14.
[0089] 2.2 Cultivation of engineering Escherichia coli
[0090] Inoculate the activated engineering Escherichia coli into the LB liquid culture solution containing chloramphenicol and ampicillin at a ratio of 1:100, and culture it with shaking...
Embodiment 3
[0099] Different fermentation conditions, such as induction temperature, rotation speed, inducer concentration, nitrogen source, substrate concentration, medium pH value and composition ratio, etc., will affect the yield of the fermentation product pinene. The present invention detects the effects of different induction temperatures, inducer concentrations and nitrogen sources on pinene output.
[0100] 3.1 Construction of E.coli recombinant strain
[0101] Transform E.coli BL21 (DE3) competent cells with pYJM27 (pACY-mvaE-mvaS-GPPS2-Pt30) and pYJM14 (pTrc-low) recombinant plasmids together by heat shock, and apply them to the antibiotics added with chloramphenicol and ampicillin The LB solid plate was obtained by PCR screening to obtain positive clones, thereby obtaining engineering Escherichia coli containing pYJM27 and pYJM14.
[0102] 3.2 Study the effect of different induction temperatures on the yield of pinene
[0103] Pick a single clone and culture it in a 5ml LB vi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com