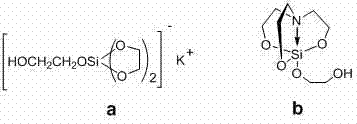Electrochemical method for synthesizing organic silicon based polymer
A basic polymer, silicone technology, applied in the direction of electrolytic organic production, electrolytic process, electrolytic components, etc., can solve the problems of high temperature, difficult to obtain silicone basic polymer, complicated post-processing, etc., to solve the problem of high energy consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In an electrolytic cell (30 mL), under nitrogen, add 0.82 g of tetrabutylammonium triflate, tetrahydrofuran (15 mL), 2.2 g (0.06875 mol) of methanol and 1.2 g (0,02 mol) of di silicon oxide. Platinum mesh was used as the working electrode, the reference electrode was silver / silver chloride, and the counter electrode was nickel mesh, and the reaction was carried out at room temperature at 2.9V for 4 hours. After the reaction was finished, it was extracted with n-pentane, and the organic solvent was removed to obtain a total of 75 grams of D3, D4 and D5. The ratio of the three is 1:5:2.
Embodiment 2
[0021] In an electrolytic cell (30 mL), under nitrogen protection, add 0.82 g of tetrabutylammonium trifluoromethanesulfonate, 1,2-dimethoxyethane (15 mL), 2.2 g (0.0478 mol) of ethanol and 1.2 grams (0,02mol) of silica. Platinum mesh was used as the working electrode, the reference electrode was silver / silver chloride, and the counter electrode was nickel mesh, and the reaction was carried out at room temperature at 2.9V for 10 hours. After the reaction was finished, it was extracted with n-pentane, and the organic solvent was removed to obtain a total of 61 grams of D3, D4 and D5. The ratio of the three is 1:4:2.
Embodiment 3
[0023] In an electrolytic cell (30 mL), under argon protection, add 0.82 g of tetrabutylammonium triflate, acetonitrile (15 mL), 3.2 g (0.1 mol) of methanol and 1.2 g (0,02 mol) of silica. A graphite plate was used as the working electrode, the reference electrode was silver / silver chloride, and the counter electrode was a nickel mesh, and 2V was applied at 0°C for 10 hours of reaction. After the reaction was finished, it was extracted with n-pentane, and the organic solvent was removed to obtain a total of 78 grams of D3, D4 and D5. The ratio of the three is 1:4:3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com