Fatty-acid-binding alhumin-drug nanoparticle lyophilized preparation and preparation method thereof
A fatty acid combination and nanoparticle technology, which is applied in pharmaceutical formulations, drug combinations, freeze-dried delivery, etc., can solve the problems of inability to effectively avoid PE38 antigenicity and reduce PE38 toxin antigenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
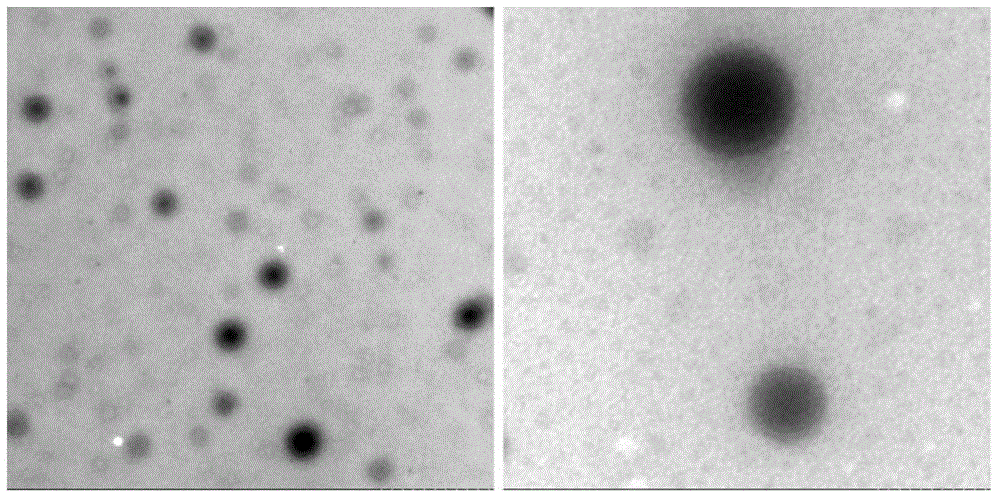
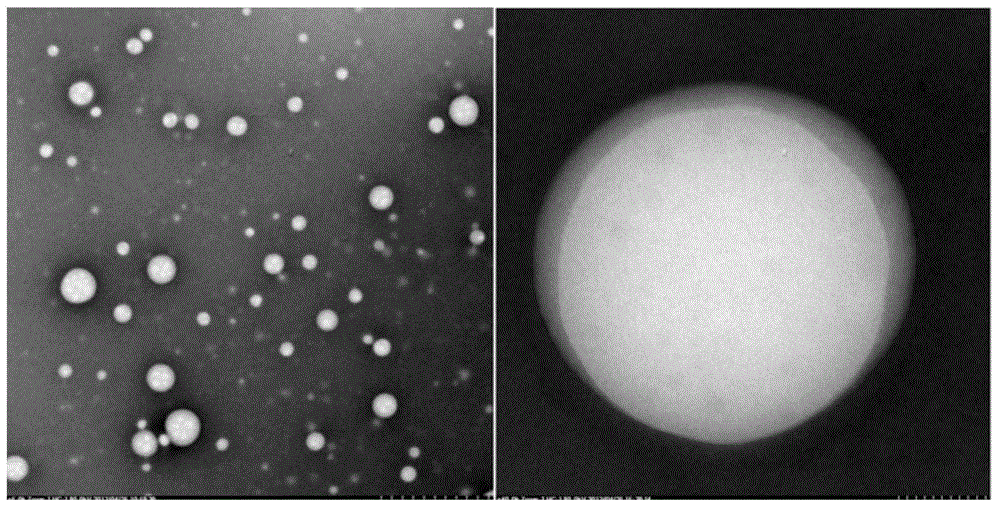
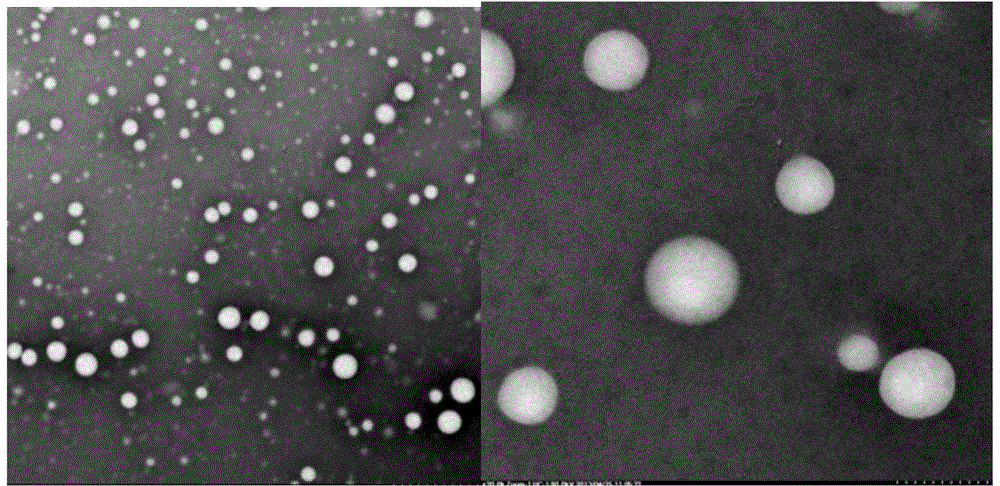
[0131] Preparation of fatty acid-binding albumin (Fatty-acid Binding Albumin, FBA)-"water-insoluble" drug (Insoluble Drug, ID) nanoparticle / solution (Solution, So) freeze-dried preparation (Nanoparticle, NP) of the present invention The method, referred to as the FBA-ID / So method, includes:
[0132] 1. "Extraction" treatment of albumin ----- preparation of liquid A and liquid C
[0133] Under sterile conditions, take human serum albumin (HSA), add water for injection, and prepare 10-200 mg / ml, preferably 20 mg-80 mg / ml HSA solution; use an organic solvent immiscible with water such as chloroform (chloroform) , dichloromethane, ethyl acetate or their mixed solution with ethanol (10~5:5~1, v / v) is liquid A, "treat" fatty acid bound to albumin molecules, organic solvent or its mixture and HSA The volume ratio of the solution is 0.3% to 15%, preferably 0.5% to 5.0%; the specific method is to slowly drop liquid A into the continuously stirred HSA solution at 5,000 to 15,000 rpm, p...
Embodiment 1
[0307] Preparation method of fatty acid-binding albumin (Fatty-acid Binding Albumin, FBA) "water-insoluble" drug (Insoluble Drug, ID) nanoparticle / solution type (Solution, So) lyophilized preparation (referred to as FBA-ID / So method)
[0308] -------FBA-ID / So method to prepare curcumin (Curcumin, C) nanoparticles freeze-dried preparation (FBA-ID / So-C)
[0309] Under sterile conditions, take 1000mg of Sigma human serum albumin (HSA), add water for injection, and prepare 10mg / ml HSA100ml; slowly drop 300ul of chloroform and absolute ethanol mixture (10:1, v / v), that is, liquid A In the 10 mg / ml HSA solution at 10,000 rpm (10,000 rpm), continue stirring for 10 minutes to obtain liquid C (reserve sample S1).
[0310] Co-dissolve 1ug of oleic acid for intravenous injection and 10mg of curcumin (Curcumin, C) in 100ul chloroform-ethanol mixed solution (9:1, v / v) (liquid D), which contains solution B, namely 1ug of oleic acid for intravenous injection / 100ul chloroform-ethanol mixed ...
Embodiment 2
[0312] Example 2: Preparation of rapamycin (Rapamycin, R) nanoparticle lyophilized formulation (FBA-ID / So-R) by FBA-ID / So method
[0313] Under sterile conditions, take 1800 mg of Sigma HSA, add water for injection, and prepare 100 mg / ml HSA 18 ml; slowly drop 500 ul of dichloromethane (liquid A) into 100 mg / ml HSA solution at 8,000 rpm for 5 minutes, and obtain liquid C ( Reserve sample S2). Dissolve 90ug of linoleic acid for intravenous injection and 20mg of rapamycin (Rapamycin, R) in 1300ul of dichloromethane solution (liquid D), and then add this solution containing linoleic acid, rapamycin and dichloromethane The mixed solution of liquid D is slowly dropped into the 100mg / ml HSA solution treated with dichloromethane under high-speed stirring, the stirring speed is 12,000rpm, and the duration is 20 minutes; this suspension is quickly put into the vacuum rotary evaporator In 35° C. under reduced pressure (20 mmHg) rotary evaporation for 30 minutes to remove all organic so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com