Escherichia coli with coexpression of L-lactate dehydrogenase and formate dehydrogenase as well as construction method and application of escherichia coli
A lactate dehydrogenase and formate dehydrogenase technology, applied in the field of Escherichia coli and its construction, can solve the problems of difficulty in obtaining high optical purity L-phenyllactic acid, the enzyme is easily affected by the external environment, and is not suitable for industrial applications, etc., to achieve High yield, simple reaction system components and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
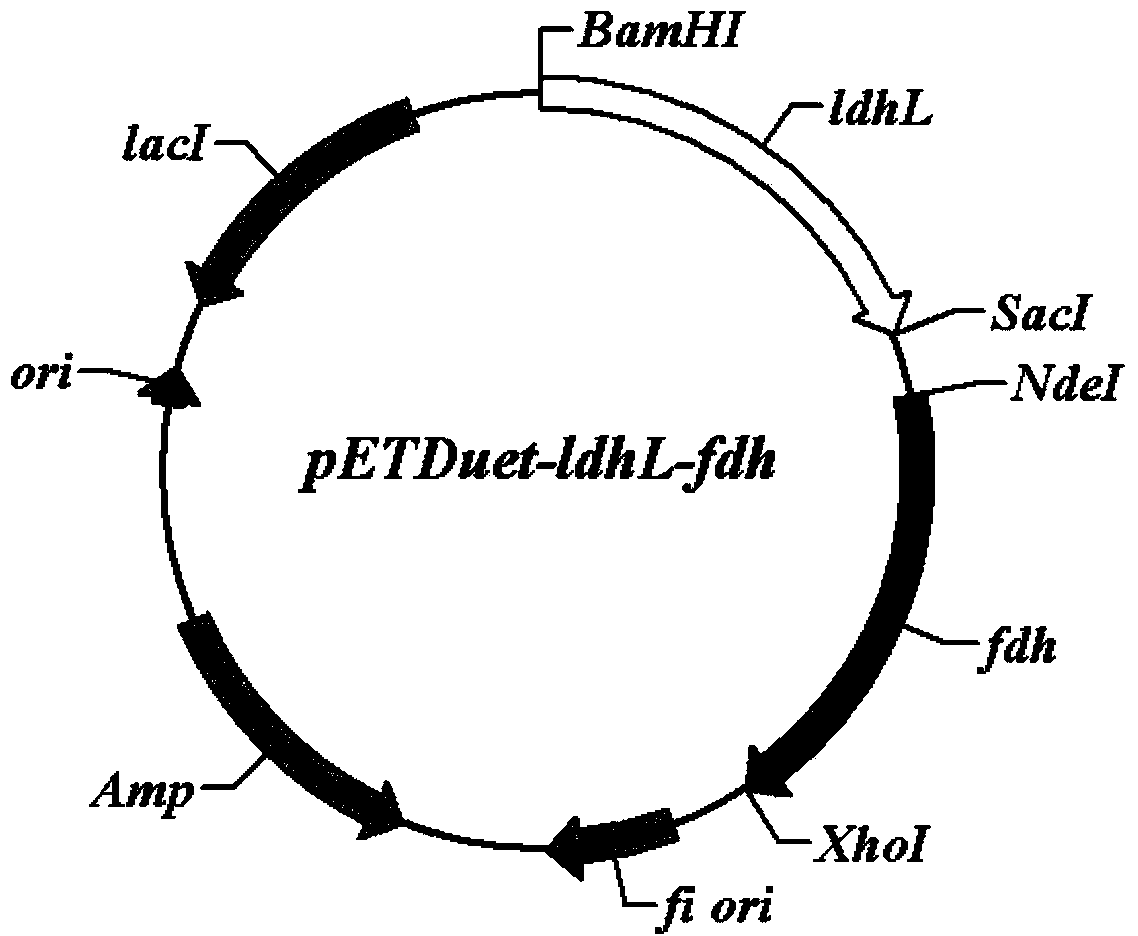
[0032] Example 1: Construction of Escherichia coli genetic engineering strain E.coli BL21 (pETDuet-ldhL-fdh).
[0033] (1) Acquisition of formate dehydrogenase gene fdh gene and construction of recombinant plasmid pETDuet-fdh.
[0034] Escherichia coli expression plasmid pETDuet-1 (Novagen) has two multiple cloning sites, which can express two target genes simultaneously. First, synthesize the formate dehydrogenase gene fdh (GenBank accession number is AJ011046) encoding Candida boidinii in the present invention by chemical synthesis, and introduce Nde that can be inserted into the multiple cloning site 2 of the pETDuet-1 plasmid at both ends of the sequence I and Xho I enzyme cutting sites, after double enzyme cutting, it was connected to the pETDuet-1 plasmid, transformed into E. coli competent cells, and after ampicillin resistance screening, the positive clone plasmid was extracted and analyzed by restriction map, and verified. Recombinant plasmid pETDuet-fdh carrying fdh...
Embodiment 2
[0042] Example 2: Coupled expression of L-lactate dehydrogenase and formate dehydrogenase in E. coli BL21 (pETDuet-ldhL-fdh).
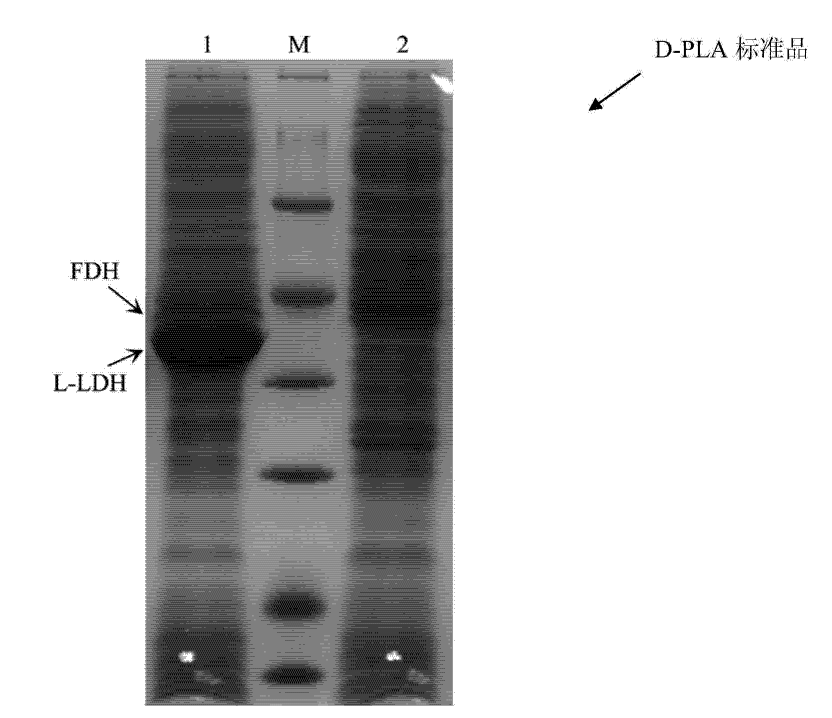
[0043] The correct recombinant strain E.coli BL21 (pETDuet-ldhL-fdh) screened in Example 1 was inoculated in 50 mL of LB liquid medium containing 100 μg / mL ampicillin, cultured on a shaker at 37°C for 12 hours, and transferred to 1 L containing 100 μg / mL ampicillin LB liquid medium, cultured on a shaker at 37°C, when the culture medium OD 600nm After reaching 0.4-0.8, IPTG with a final concentration of 1 mmol / L was added to induce expression, and culture was continued for 5 hours at 25°C. After the induction culture was over, centrifuge at 12,000 rpm for 5 minutes to obtain cell pellets, wash twice with saline, and resuspend in 100 mL of phosphate buffer with pH 7.4 and 50 mmol / L. Ultrasound was used to break the cell wall in the ice-water mixture. The breaking time was 30 minutes. The solution after breaking the wall was centrifuged at 16,000 rpm f...
Embodiment 3
[0044] Example 3: E.coli BL21 (pETDuet-ldhL-fdh) produces L-phenyllactic acid under different pH conditions.
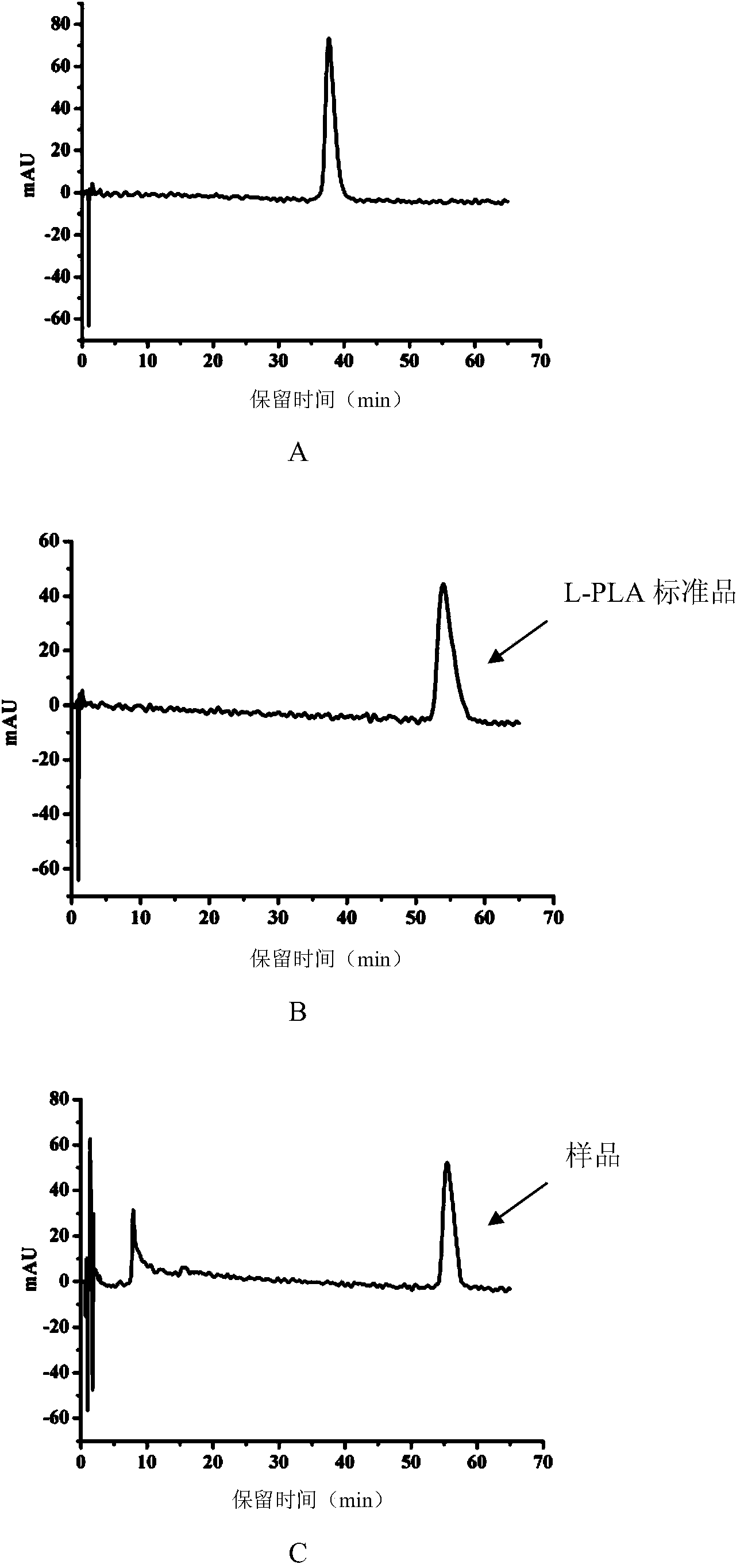
[0045] In the 10mL reaction system, add 50mmol / L substrate phenylpyruvate, 100mmol / L auxiliary substrate sodium formate, and recombinant Escherichia coli wet cells with a dry weight of 7g / L, mix well and shake the reaction on a constant temperature shaker at 37°C , the pH of the reaction system was respectively 5.0, 5.5, 6.0, 6.5, 7.0, 7.5. After 1 hour, the reaction mixture was centrifuged to remove the thallus, and the supernatant liquid was taken to detect the output and optical purity of L-phenyllactic acid generated. The results showed (Table 1), when the pH of the reaction system is 5.5-6.0, the yield of L-phenyllactic acid is the highest, which is 40.1 mmol / L, and the optical purity of L-phenyllactic acid is greater than 99.9%.
[0046] The output of L-phenyllactic acid under different pH conditions in table 1
[0047] pH
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com