Carbon-decorated porous lithium vanadium phosphate nanosphere material as well as preparation method and application thereof
A technology of lithium vanadium phosphate and nanospheres, which is applied in the field of nanomaterials and electrochemistry, can solve problems such as complex preparation methods, electrochemical performance needs to be improved, and is not conducive to material industrialization, and achieves simple process, favorable for market promotion, and easy Amplified effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
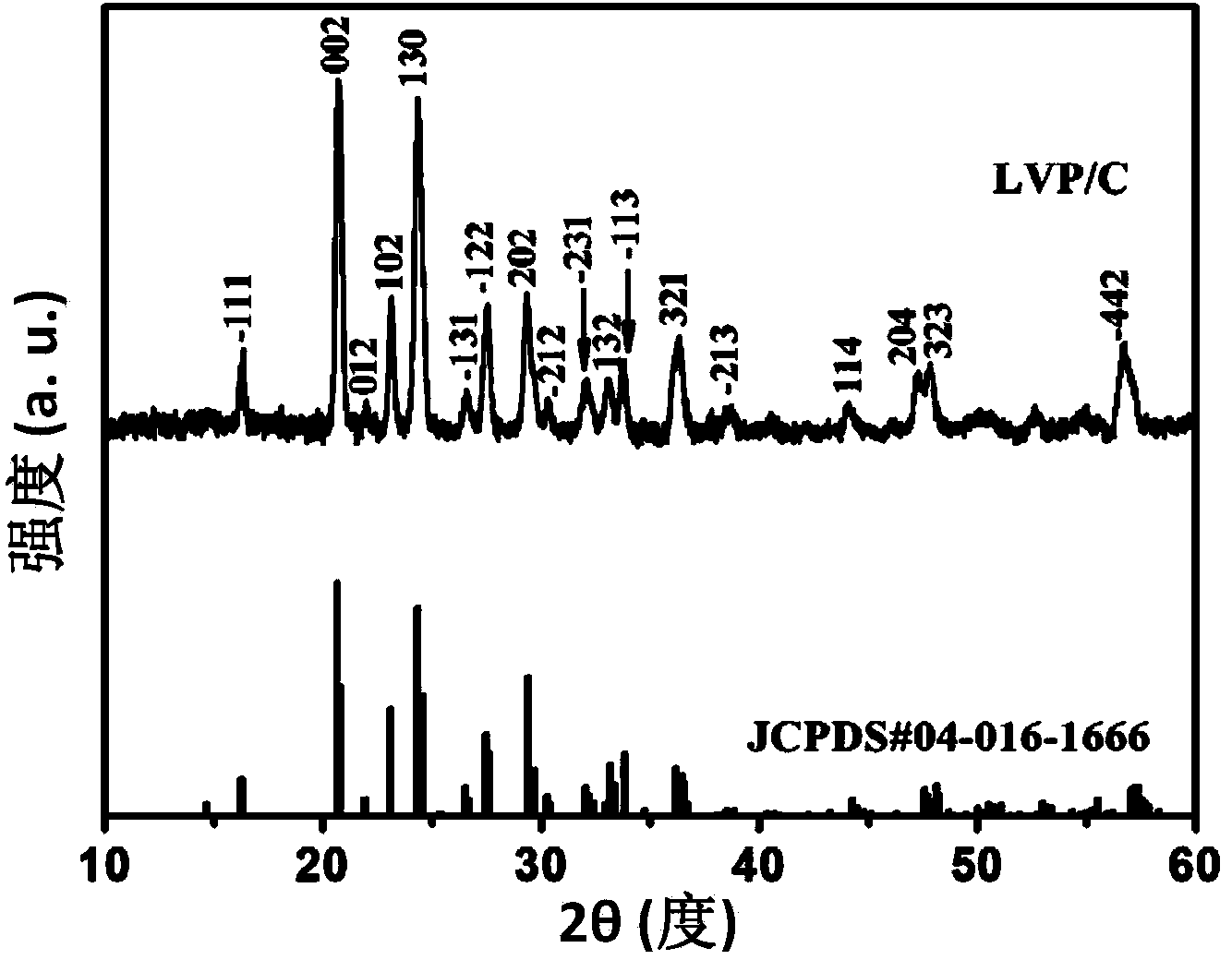
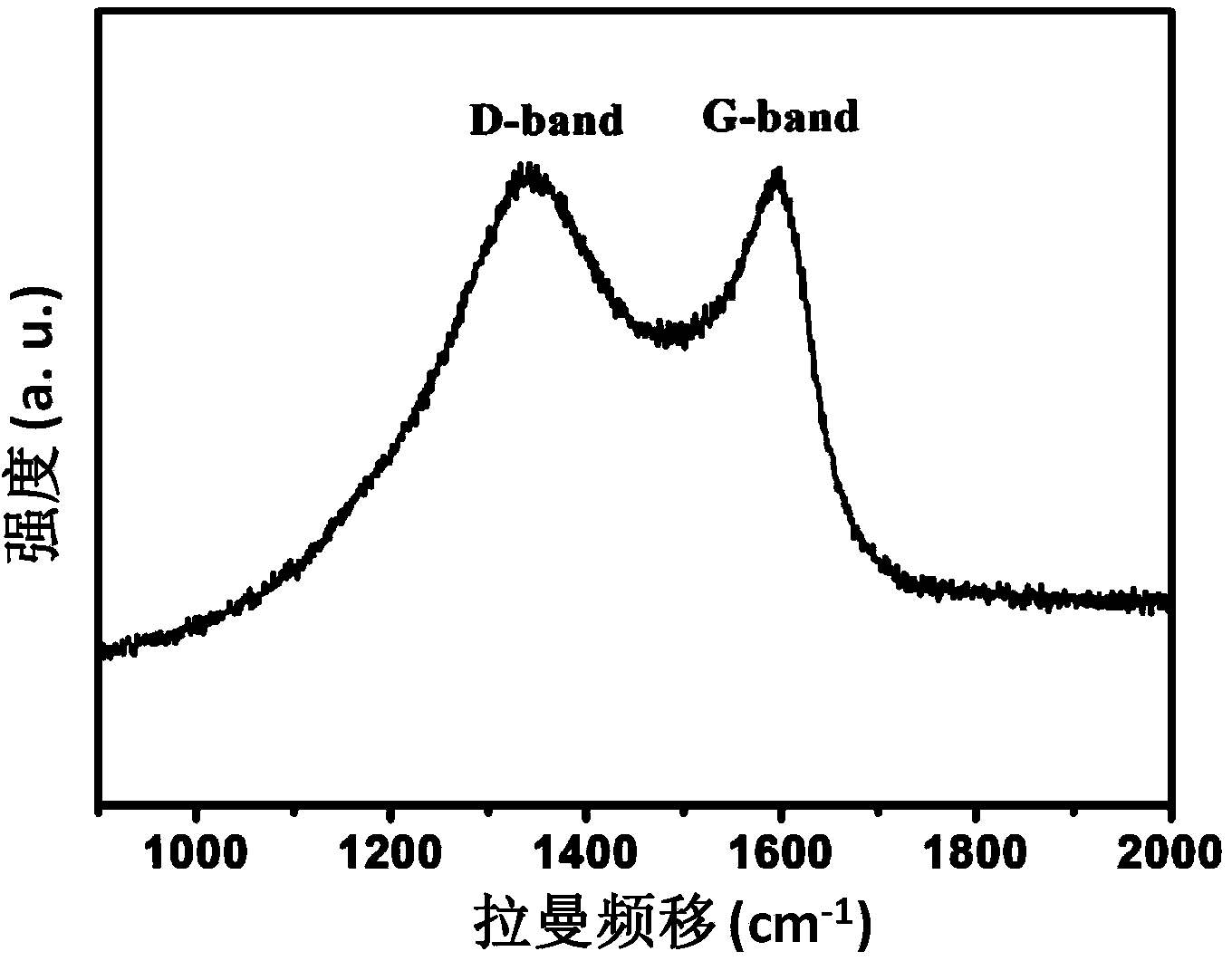
Image
Examples
Embodiment 1
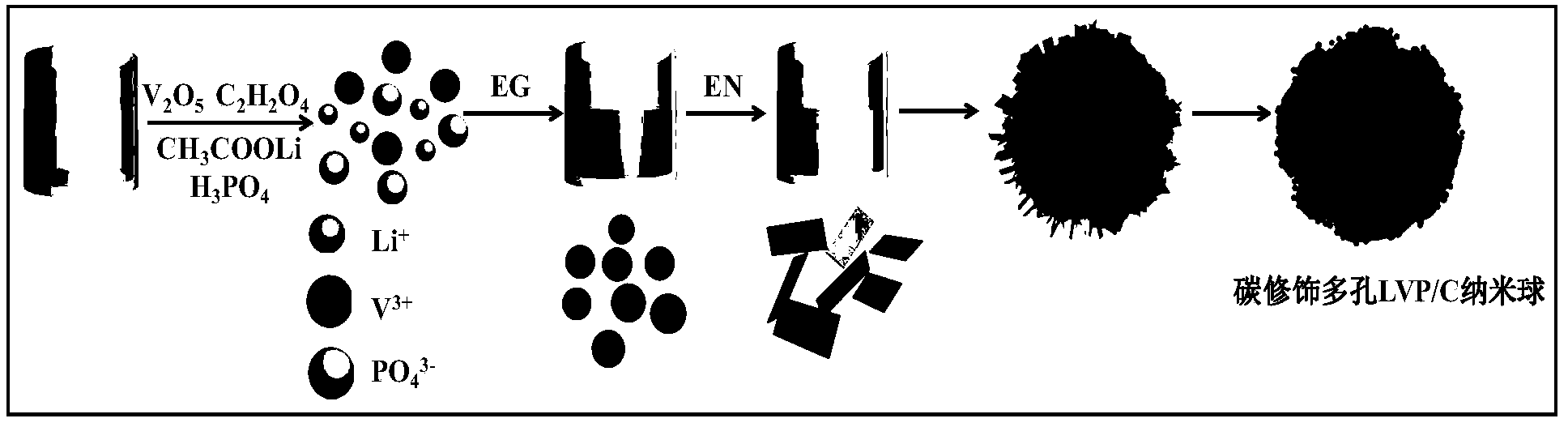
[0035] The preparation method of carbon modified porous lithium vanadium phosphate nanosphere material, such as figure 1 As shown, it includes the following steps:
[0036] 1) 1.78 vanadium pentoxide (V 2 o 5 ) and 3.696g oxalic acid (C 2 h 2 o 4 ) into 40mL distilled water (V 2 o 5 The molar ratio of oxalic acid and oxalic acid is 1:3), mixed and stirred at 80°C for 10 minutes to obtain VOC 2 o 4 blue solution;
[0037] 2) Measure 85% phosphoric acid (H 3 PO 4 ) solution (2mL), phosphoric acid was dripped dropwise into the blue solution of step 1) gained, and stirred evenly;
[0038] 3) Weigh 3.142g of lithium acetate dihydrate (LiAc, the actual amount of lithium source is 1.05 times the required reaction amount) powder, dissolve it in 30mL of distilled water, and drop it into the blue solution obtained in step 2) after dissolving;
[0039] 4) Weigh 5mL of ethylene glycol with a molar ratio of 1:9.12 to vanadium pentoxide, add dropwise to the solution obtained in ...
Embodiment 2
[0050] 1) 1.78 vanadium pentoxide (V 2 o 5 ) and 3.696g oxalic acid (C 2 h 2 o 4 ) into 40mL distilled water (V 2 o 5 The molar ratio of oxalic acid and oxalic acid is 1:3), mixed and stirred at 80°C for 10 minutes to obtain VOC 2 o 4 blue solution;
[0051] 2) Weigh ammonium dihydrogen phosphate (NH 4 h 2 PO 4 ) 3.38g, was dissolved in 10mL distilled water, and the solution was dripped dropwise into the blue solution obtained in step 1), and stirred evenly;
[0052] 3) Weigh 1.14g lithium carbonate (Li 2 CO 3 , the actual amount of lithium source is 1.05 times of the required reaction amount) powder, dissolved in 30mL of distilled water, and dropped into the blue solution obtained in step 2) after dissolving;
[0053] 4) Weigh 4.39 mL of ethylene glycol with a molar ratio of 1:8 to vanadium pentoxide, add dropwise to the solution obtained in step 3), and stir evenly;
[0054] 5) Weigh 3.93mL of ethylenediamine with a molar ratio of 1:6 to vanadium pentoxide, add...
Embodiment 3
[0060] 1) 1.78 vanadium pentoxide (V 2 o 5 ) and 3.696g oxalic acid (C 2 h 2 o 4 ) into 40mL distilled water (V 2o 5 The molar ratio of oxalic acid and oxalic acid is 1:3), mixed and stirred at 80°C for 10 minutes to obtain VOC 2 o 4 blue solution;
[0061] 2) Weigh ammonium dihydrogen phosphate (NH 4 h 2 PO 4 ) 3.38g, was dissolved in 10mL distilled water, and the solution was dripped dropwise into the blue solution obtained in step 1), and stirred evenly;
[0062] 3) Weigh 2.13g lithium nitrate (LiNO 3 , the actual amount of lithium source is 1.05 times of the required reaction amount) powder, dissolved in 30mL of distilled water, and dropped into the blue solution obtained in step 2) after dissolving;
[0063] 4) Weigh 5.48 mL of ethylene glycol with a molar ratio of 1:10 to vanadium pentoxide, add dropwise to the solution obtained in step 3), and stir evenly;
[0064] 5) Weigh 5.89mL of ethylenediamine with a molar ratio of 1:9 to vanadium pentoxide, add dropw...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge specific capacity | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com