Polysubstituted quinazoline imine derivative and preparation method thereof
A technology for quinazoline imine and derivatives, which is applied in the field of polysubstituted quinazoline imine derivatives and their preparation, can solve the problems of reduced reaction yield, difficulty in preparation, harm and the like, and achieves high yield and synthesis. The method is simple, the synthesis method is scientific and reasonable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
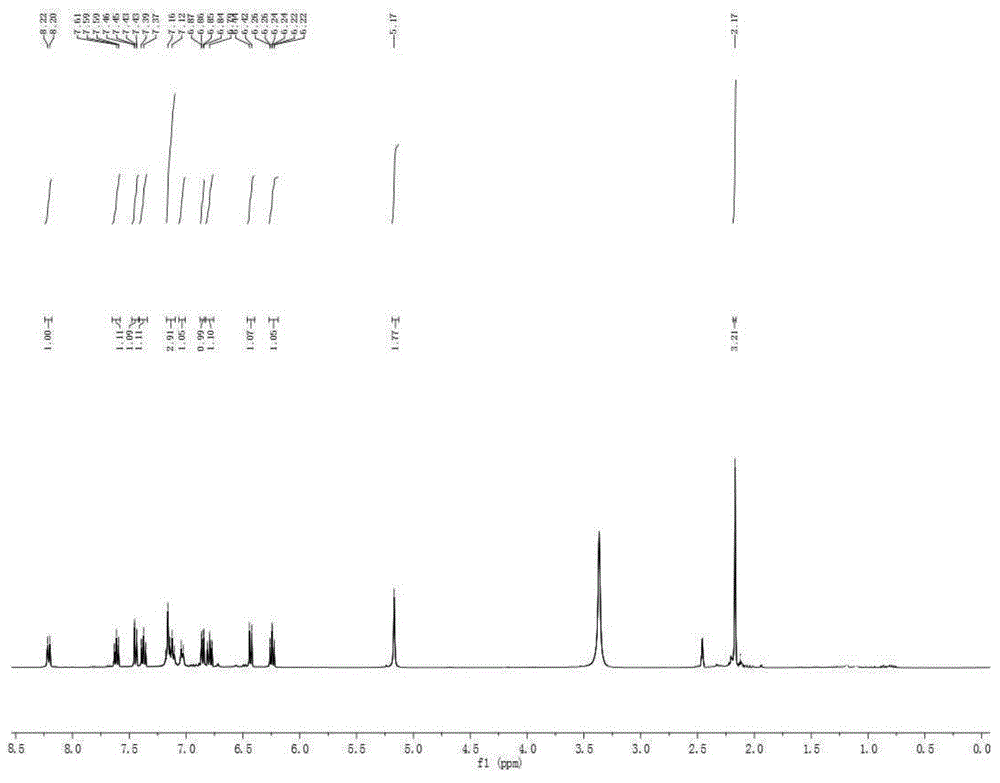
[0035] The preparation of embodiment 1,2-(3-phenyl)-4-imino-3,4-dihydroquinazoline)-2-aniline (structural formula (II))
[0036]
[0037] Into a 25mL sealed tube, add 2-aminobenzonitrile (2.0mmol, 236mg), diphenyl hypervalent iodine hexafluorophosphate (1.0mmol, 426mg), replace nitrogen three times, add 1,2-dichloroethane alkanes (3 mL). The system was stirred at room temperature for 15-30 minutes to make the system evenly mixed, and the sealed tube was moved into an oil bath at 120°C to react for 2 days. After the reaction, the system was cooled to room temperature. Add 3mL of methanol, potassium carbonate (1mmol, 138mg), 2 drops of deionized water to the system, and stir at room temperature for 3-5h. The system solution was concentrated and spin-dried to obtain a crude product. The crude product was separated by column chromatography (200-300 mesh silica gel) with ethyl acetate:petroleum ether:triethylamine=5:3:1 as the eluent, and the milky white solid product 2-(3- ...
Embodiment 2
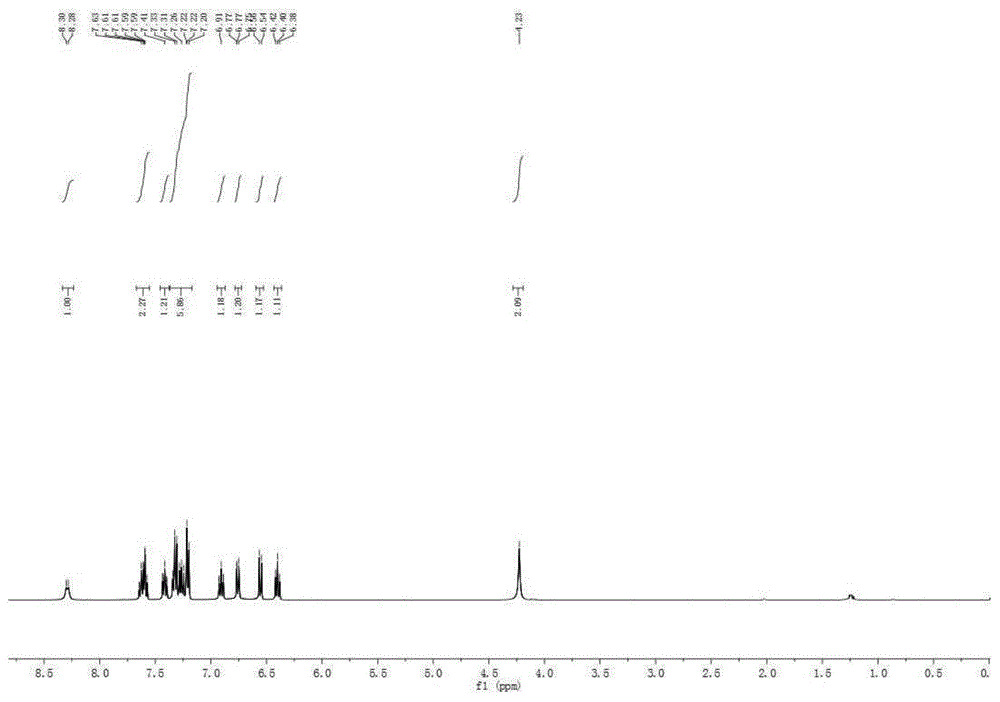
[0044] Embodiment 2, the preparation of 2-(3-(4-methylbenzene)-4-imino-3,4-dihydroquinazoline)-2-aniline (structural formula (III))
[0045]
[0046] Into a 25mL sealed tube, add 2-aminobenzonitrile (2.0mmol, 236mg), bis(3-methyl-phenyl) hypervalent iodine hexafluorophosphate (1.0mmol, 426mg), replace nitrogen three times, add 1,2-Dichloroethane (3 mL). The system was stirred at room temperature for 15-30 minutes to make the system evenly mixed, and the sealed tube was moved into an oil bath at 120°C to react for 2 days. After the reaction, the system was cooled to room temperature. Add 3mL of methanol, potassium carbonate (1mmol, 138mg), 2 drops of deionized water to the system, and stir at room temperature for 3-5h. The system solution was concentrated and spin-dried to obtain a crude product. The crude product was separated by column chromatography (200-300 mesh silica gel) with ethyl acetate:petroleum ether:triethylamine=5:3:1 as eluent, and the brown solid product 2...
Embodiment 3
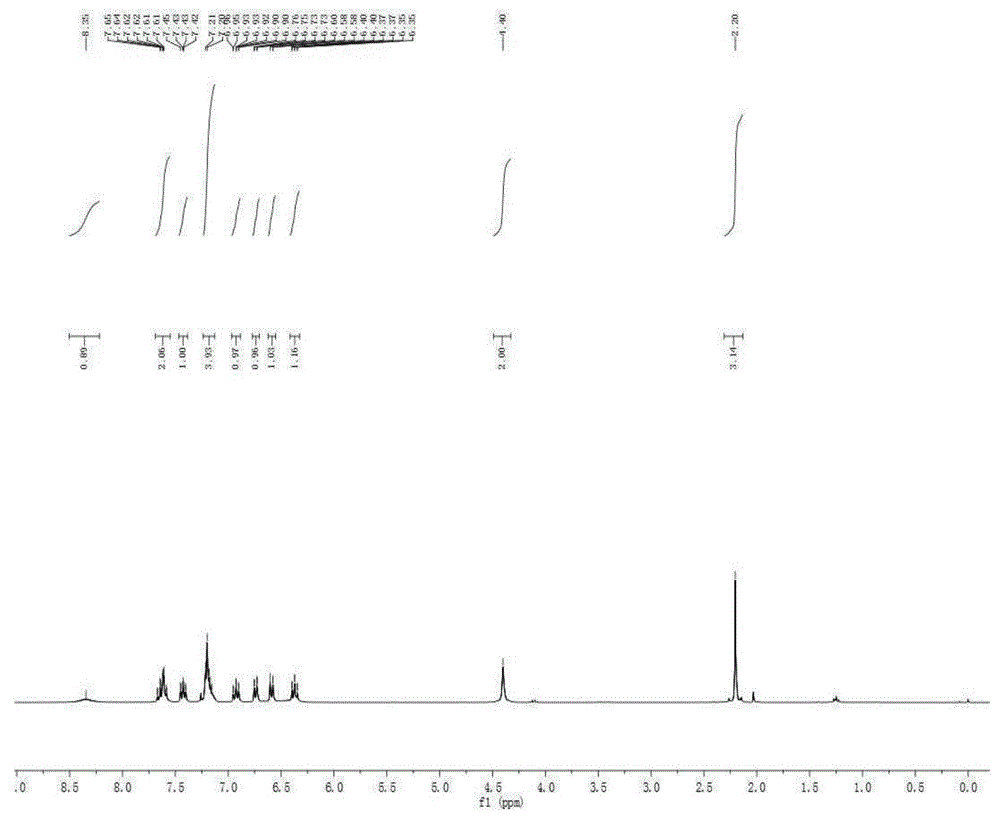
[0053] Embodiment 3, the preparation of 2-(3-(3-methylbenzene)-4-imino-3,4-dihydroquinazoline)-2-aniline
[0054] Into a 25mL sealed tube, add 2-aminobenzonitrile (2.0mmol, 236mg), bis(3-methyl-phenyl) hypervalent iodine hexafluorophosphate (1.0mmol, 426mg), replace nitrogen three times, add 1,2-Dichloroethane (3 mL). The system was stirred at room temperature for 15-30 minutes to make the system evenly mixed, and the sealed tube was moved into an oil bath at 120°C to react for 2 days. After the reaction, the system was cooled to room temperature. Add 3mL of methanol, potassium carbonate (1mmol, 138mg), 2 drops of deionized water to the system, and stir at room temperature for 3-5h. The system solution was concentrated and spin-dried to obtain a crude product. The crude product was separated by column chromatography (200-300 mesh silica gel) with ethyl acetate:petroleum ether:triethylamine=5:3:1 as eluent, and the yellow solid product 2-(3- Preparation of (3-methylbenzene)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com