Lithium manganese phosphate nanoparticles and preparation method thereof
A nanoparticle, lithium manganese phosphate technology, applied in nanotechnology, nanotechnology, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
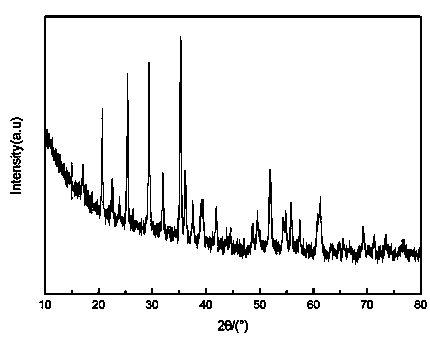
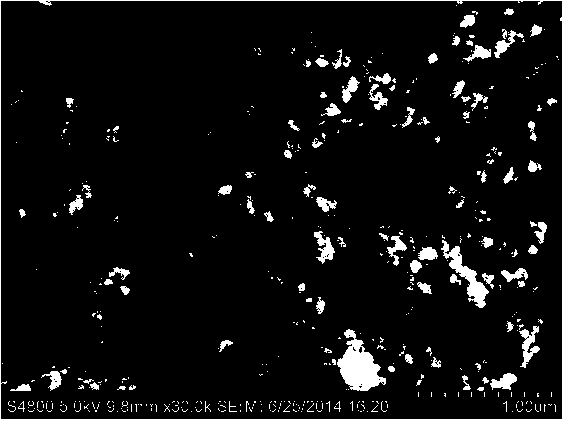
Image
Examples
example 1
[0021] 1) Dissolve 2.000 g of P123 in 15 ml of deionized water, stir for 240 minutes, then add 1.470 g of manganese acetate tetrahydrate and 0.200 g of ascorbic acid, and stir until fully dissolved to obtain a manganese acetate concentration of 0.4 mol / L, ascorbic acid Solution A with a concentration of 0.076 mol / L and a P123 concentration of 0.13 g / mL;
[0022] 2) Dissolve 0.588g of phosphoric acid and 0.612g of lithium acetate dihydrate in 15 ml of ethylene glycol and stir for 30 minutes to form a suspension with a phosphoric acid concentration of 0.4 mol / L and a lithium acetate concentration of 0.4 mol / L B;
[0023] 3) Add the suspension B prepared in step 2) dropwise to the solution A prepared in step 1) under stirring to form emulsion C. The molar ratio of Li, Mn, and P in emulsion C is 1:1:1.
[0024] 4) Transfer the emulsion C in step 3) to a 60ml autoclave, add 5 ml of ethylene glycol solution containing 0.112 g KOH, stir well, and then adjust its volume to 40ml with...
example 2
[0028] 1) Dissolve 3.000 g P123 in 15 ml of deionized water, stir for 300 minutes, then add 2.45 g of manganese acetate tetrahydrate and 0.400 g of ascorbic acid, and stir until fully dissolved to obtain a manganese acetate concentration of 0.67 mol / L, ascorbic acid Solution A with a concentration of 0.152 mol / L and a P123 concentration of 0.20 g / mL;
[0029] 2) Dissolve 0.98g of phosphoric acid and 2.04g of lithium acetate dihydrate in 15 ml of ethylene glycol and stir for 90 minutes to form a suspension with a concentration of phosphoric acid of 0.67 mol / L and a concentration of lithium acetate of 1.33 mol / L B;
[0030] 3) Add the suspension B prepared in step 2) dropwise to the solution A prepared in step 1) under stirring to form emulsion C. The molar ratio of Li, Mn, and P in emulsion C is 2:1:1.
[0031] 4) Transfer the emulsion C in step 3) to a 50ml autoclave, add 5 ml of ethylene glycol solution containing 0.224 g KOH, stir well, and then adjust its volume to 40ml w...
example 3
[0034] 1) Dissolve 3.500 g of P123 in 15 ml of deionized water, stir for 360 minutes, then add 2.94 g of manganese acetate tetrahydrate and 0.240 g of ascorbic acid, stir until fully dissolved, and obtain manganese acetate concentration of 0.80 mol / L, ascorbic acid Solution A with a concentration of 0.091 mol / L and a P123 concentration of 0.23 g / mL;
[0035] 2) Dissolve 1.176g of phosphoric acid and 1.224g of lithium acetate dihydrate in 15 ml of ethylene glycol and stir for 150 minutes to form a suspension with a phosphoric acid concentration of 0.80 mol / L and a lithium acetate concentration of 0.80 mol / L B;
[0036] 3) Add the suspension B prepared in step 2) dropwise to the solution A prepared in step 1) under stirring to form emulsion C. The molar ratio of Li, Mn, and P in emulsion C is 1:1:1.
[0037] 4) Transfer the emulsion C in step 3) to a 55ml autoclave, add 8 ml of ethylene glycol solution containing 0.336 g KOH, stir well, and then adjust its volume to 40ml with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com