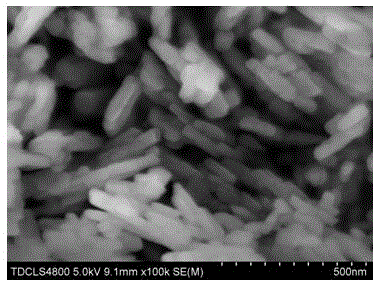
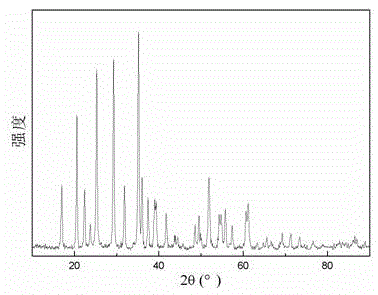
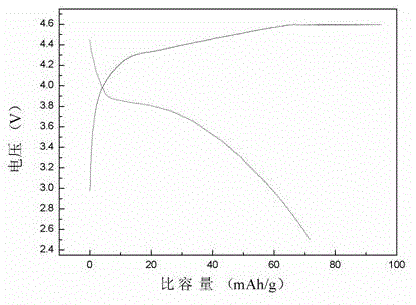
Preparation method of lithium manganese phosphate nanorod
A lithium manganese phosphate and nanorod technology is applied in the field of positive electrode materials of lithium ion batteries, which can solve the problems of large particle size, which is not conducive to improving the energy density of lithium ion batteries, and achieves good dispersibility, which is conducive to lithium ion diffusion, Easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Weigh 2.2058g of manganese acetate tetrahydrate and dissolve it in 15mL of ethylene glycol and stir for 0.5h, add 0.6273g of sodium dodecylbenzenesulfonate and stir for 1h to obtain manganese acetate sodium dodecylbenzenesulfonate solution A. Another weighed 0.378g of lithium hydroxide monohydrate was dissolved in 15mL of ethylene glycol and stirred for 0.5h to obtain solution B. Add solution B to solution A and stir for 0.5h to obtain a mixed solution. Finally, add Stir 1.038g of phosphoric acid with a mass concentration of 85% for 1 hour to obtain a precursor solution, then transfer it to a polytetrafluoroethylene reaction kettle, seal it, keep it at 200°C for 10 hours, then cool it down to room temperature, take out the resulting solution, and filter it out. After the supernatant is obtained, add deionized water for ultrasonic oscillation, and then use a centrifuge to centrifuge at a speed of 10,000 rpm for 10 minutes. After filtering off the supernatant, add deionize...
Embodiment 2
[0017] Weigh 2.2058g of manganese acetate tetrahydrate and dissolve it in 15mL of ethylene glycol and stir for 0.5h, add 0.6273g of sodium dodecylbenzenesulfonate and stir for 1h to obtain manganese acetate sodium dodecylbenzenesulfonate solution A. Another weighed 0.378g of lithium hydroxide monohydrate was dissolved in 15mL of ethylene glycol and stirred for 0.5h to obtain solution B. Add solution B to solution A and stir for 0.5h to obtain a mixed solution. Finally, add Stir 1.038g of phosphoric acid with a mass concentration of 85% for 1 hour to obtain a precursor solution, then transfer it to a polytetrafluoroethylene reaction kettle, seal it, keep it at 200°C for 16 hours, then cool it down to room temperature, take out the resulting solution, and filter it out. After the supernatant is obtained, add deionized water for ultrasonic oscillation, and then use a centrifuge to centrifuge at a speed of 10,000 rpm for 10 minutes. After filtering off the supernatant, add deionize...
Embodiment 3
[0019] Weigh 0.7353g of manganese acetate tetrahydrate, dissolve it in 15mL of ethylene glycol and stir for 0.5h, add 0.2091g of sodium dodecylbenzene sulfonate and stir for 1h to obtain the sodium dodecylbenzenesulfonate solution A of manganese acetate. Another weighed 0.126g of lithium hydroxide monohydrate was dissolved in 15mL of ethylene glycol and stirred for 0.5h to obtain solution B. Add solution B dropwise to solution A and stir for 0.5h to obtain a mixed solution. Finally, add Stir 0.346g of phosphoric acid with a mass concentration of 85% for 1 hour to obtain a precursor solution, then transfer it to a polytetrafluoroethylene reaction kettle, seal it, keep it warm at 200°C for 10 hours, then cool it down to room temperature, take out the resulting solution, and filter it out. After the supernatant is obtained, add deionized water for ultrasonic oscillation, and then use a centrifuge to centrifuge at a speed of 10,000 rpm for 10 minutes. After filtering off the supern...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com