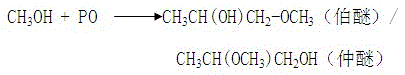A kind of method preparing dipropylene glycol methyl ether
A technology of dipropylene glycol methyl ether and propylene glycol methyl ether, which is applied in the field of preparing dipropylene glycol methyl ether, can solve the problems of energy consumption and low market value of 2-methoxy-1-propanol, and achieve the effect of saving energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A kind of method preparing dipropylene glycol methyl ether, concrete steps are as follows:
[0049] (1) With the reaction of the tower still liquid after having extracted propylene glycol methyl ether and propylene oxide, specifically:
[0050] (1a) The mass fraction of water in a certain batch of tower bottom liquid used as raw material was measured by Karl Fischer moisture meter to be 0.05%; the mass fraction of sodium methoxide in the tower bottom liquid was determined to be 2.68 by the volumetric method of acid-base titration %; use 25m × 0.32mm × 0.5μm FFAP strong polar capillary column to analyze on the gas chromatograph, after multiplying the area percentage of each component by (1-mass fraction of water-mass fraction of sodium methoxide), The mass fraction of some components that can be detected by gas chromatography in this batch of tower bottom liquid that obtains is:
[0051] Propylene Glycol Methyl Ether 0.15%
[0052] 2-methoxy-1-propanol 77.67%
[0053]...
Embodiment 2
[0088] On the basis of embodiment 1, other conditions are all the same, just change the mass fraction of 98.5% sodium hydroxide into a mass fraction of 97.2% granular solid sodium methoxide, and the quality of sodium methoxide added in step (1a) is still 2.3g, but the molar number of sodium methoxide added is the total moles of propylene glycol methyl ether and 2-methoxyl-1-propanol Count 0.010 times of 4 mol, and finally obtain 1900 mL of dipropylene glycol methyl ether.
[0089] Verification test: using FFAP strong polar capillary chromatographic column, the sum of the mass fractions of the four isomers of dipropylene glycol methyl ether measured on the gas chromatograph is 97.7%.
[0090] Take 100mL dipropylene glycol methyl ether sample, and use a special device for measuring the boiling range to measure the boiling range of dipropylene glycol methyl ether as 182.6-194.4°C.
[0091] The boiling ranges of dipropylene glycol methyl ether obtained in the above two examples a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com