Urease inhibitor determination method based on fluorescence gold nano cluster
A urease inhibitor, fluorescent gold nanotechnology, applied in analytical chemistry and nanometer fields, can solve problems such as economic loss and environmental pollution, and achieve the effect of simple and fast preparation process and inhibition of fluorescence quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0030] Add 0.6 mL of 0.5 mol / L sodium hydroxide solution and 0.4 mL of 0.02 g / L chloroauric acid solution to 4 mL of 0.08 mol / L N-acetyl-L-cysteine solution , mixed well, and placed in a 37°C constant temperature water bath to react for 2.5 h. After the reaction, the reaction solution was purified with a dialysis bag with a molecular weight cut off of 3500. The obtained gold nanocluster solution is colorless under visible light, and produces strong red fluorescence under ultraviolet light irradiation. Stored in the dark at 4°C, it can remain relatively stable for at least one month.
example 2
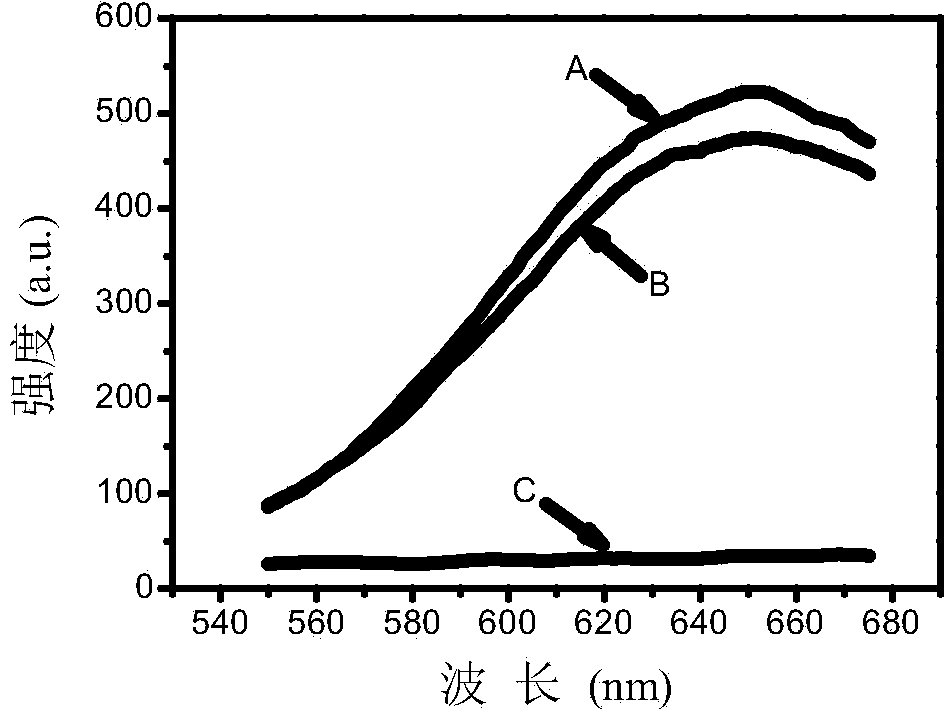
[0032]Add 0.05 mL of urease solution (pH=6.0) with a concentration of 1.5 U / mL to 0.2 mL of urease solution containing mercaptoethylamine (8 μmol / L) or p-benzoquinone (50 μmol / L) at a concentration of 1 mol / L In urea solution (pH=6.0), after mixing evenly, react in a constant temperature water bath at 25°C for 40 min. (2) Add 0.2 mL of the solution prepared in Example 1 to the above reaction solution, and react in a constant temperature water bath at 25°C for 3 min. Set up a group of blank control groups without urease inhibitors. After the reaction was completed, observe under a UV lamp, and add 8 μmol / L mercaptoethylamine ( figure 1 A in A) or 50 μmol / L p-benzoquinone ( figure 1 After B), the gold nanoclusters have red fluorescence, while the red fluorescence of the control group is quenched ( figure 1 in C). figure 2 is the corresponding fluorescence emission spectrum.
example 3
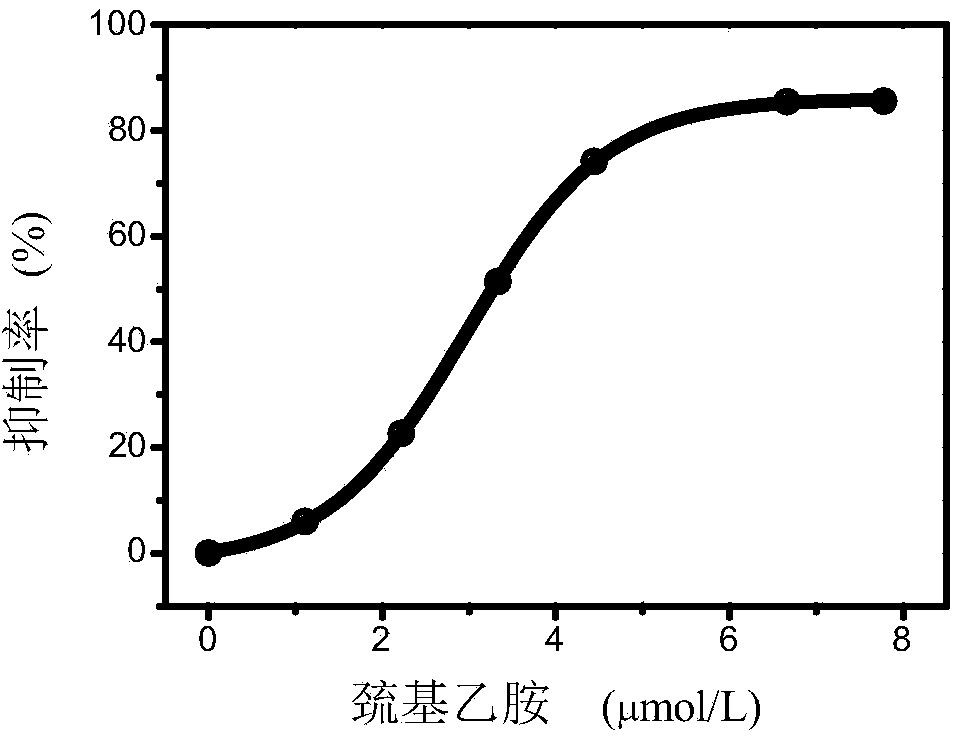
[0034] Add 0.05 mL of urease solution (pH=6.0) with a concentration of 1.5 U / mL into 0.2 mL of urea solution (pH=6.0) with a concentration of 1 mol / L containing different concentrations of mercaptoethylamine. C for 40 min in a constant temperature water bath. Add 0.2 mL of the solution prepared in Example 1 to the above reaction solution, react in a constant temperature water bath at 25°C for 3 min, and measure the emitted light intensity value F 650 value to calculate the inhibition rate. The result is as image 3 As shown, the IC of mercaptoethylamine was obtained by software fitting 50 It is 2.8 μmol / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com