Application of a tetraphenylethylene derivative in the preparation of antibacterial drugs
A technology of tetraphenylethylene and antibacterial drugs, which is applied in the field of tetraphenylethylene derivatives in the preparation of antibacterial drugs, can solve the problems of epidemics of infectious diseases, pollution, etc., and achieve low cytotoxicity, broad application prospects, and no hemolytic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]Embodiment 1: the synthesis of tetraphenylethylene derivative
[0049] Taking four-p-(p-carboxyphenyl) tetraphenylethylene as an example, its synthetic method is as follows:
[0050]
[0051] The specific steps are:
[0052] 1) Place 5g of compound 1 on the upper layer of a clean desiccator, spread about 7.5mL of bromine on the bottom of the desiccator, react for one week, dissolve the reacted solid in dichloromethane, and use methanol as a poor solvent for recrystallization. A total of 8.2 g of compound 2 was obtained by suction filtration.
[0053] 2) 644mg (1mmol) compound 2, 1.44g (8mmol) compound 3, 70mg (0.05mmol) Pd (PPh 3 ) 4 , 190mg TBAB, 5mL of 2M K 2 CO 3 The solution and 35mL THF were added into the reaction flask, heated to reflux, under nitrogen protection, and the reaction temperature was 70°C. After reacting for 48 hours, it was cooled to room temperature, washed with water three times, and the organic phase was collected. Mixed with silica gel,...
Embodiment 2
[0056] Example 2: Therapeutic effect of tetraphenylethylene derivatives on animals infected with multidrug-resistant bacteria and common bacteria
[0057] First put 10 6 CFU / mL multidrug-resistant bacteria 500 μL was injected into the abdominal cavity of mice to simulate the abdominal infection model. Normal saline was used as negative control, and vancomycin was used as positive control. Half an hour after the modeling, 100 μL of the normal saline solution of tetraphenylethylene derivatives, the negative control and the positive control were injected into the tail vein, and 100 μL was injected again 8 hours later. Observe and record the survival rate of mice. The measurement results are shown in Table 1.
[0058] Table 1
[0059]
[0060] It can be seen that in the case of all death of animals without medication, the tetraphenylethylene derivatives of 400 μg / kg dose can make the animals infected with multi-drug resistant bacteria obtain 100% survival rate, which has th...
Embodiment 3
[0062] Example 3: Inhibition of Tetraphenylethylene Derivatives to Planktonic Bacteria
[0063] The inhibitory effect of tetraphenylethylene derivatives on planktonic bacteria was expressed by the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) against planktonic bacteria in vitro.
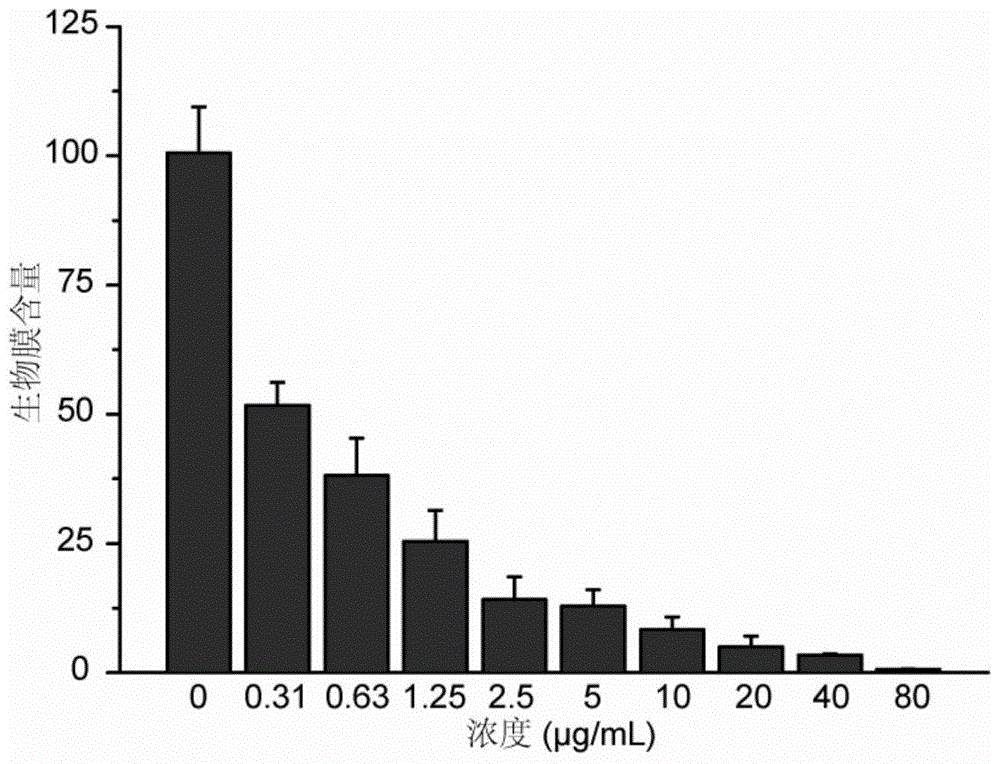
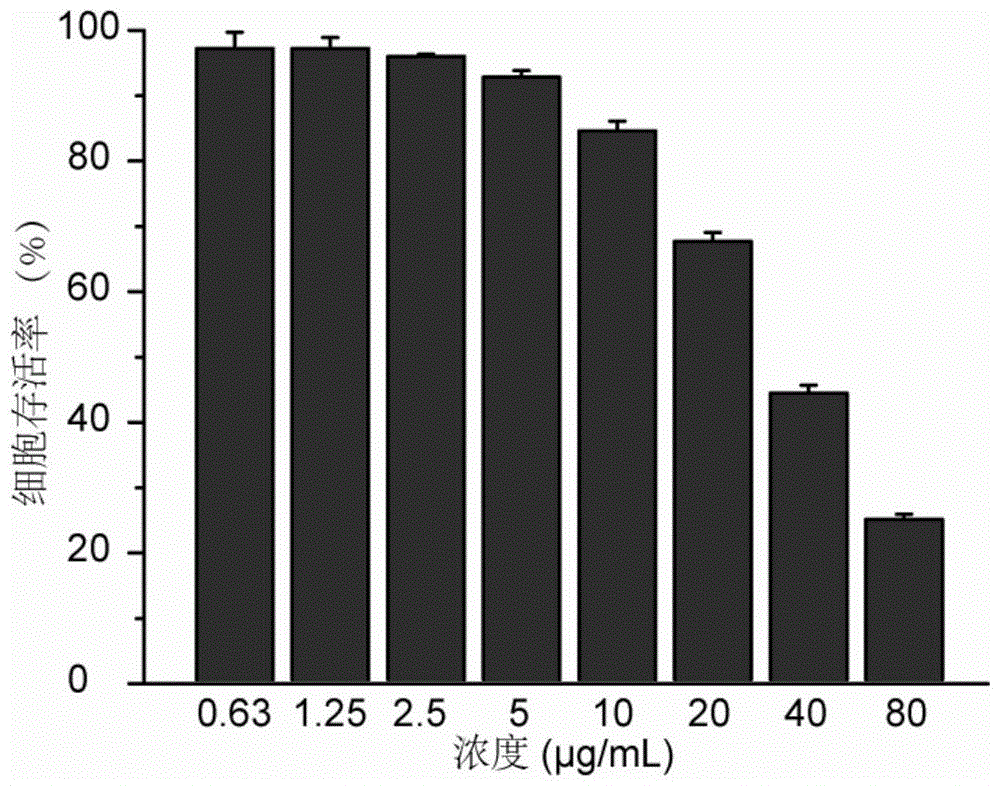
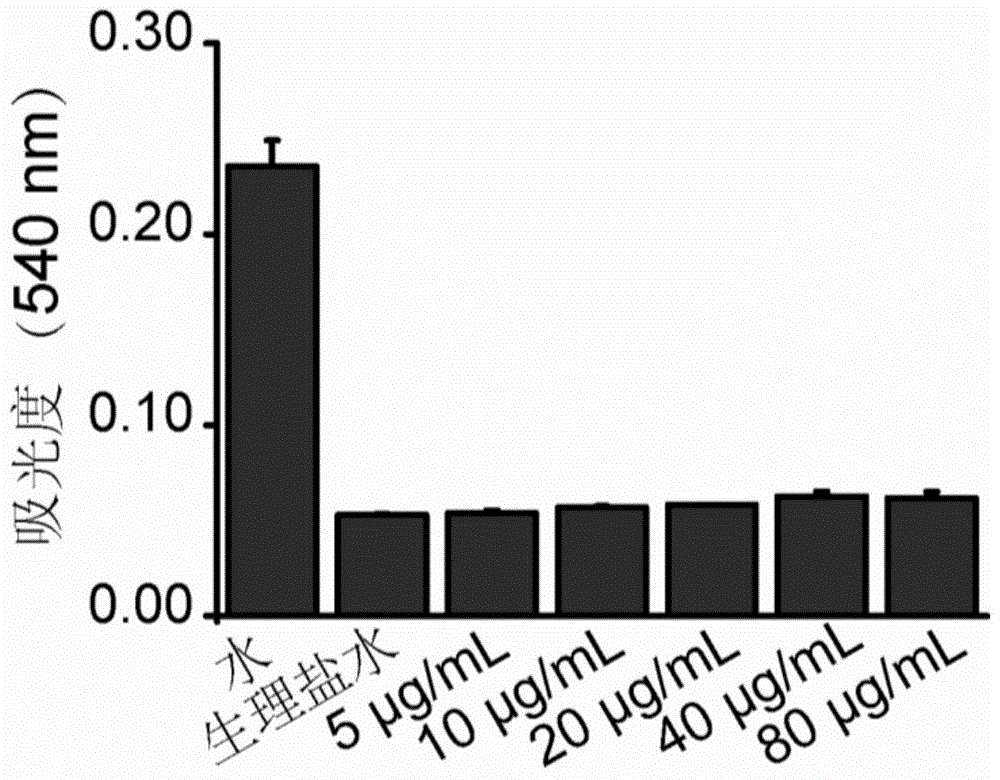
[0064] Five different bacteria S.aureus, MRSA, S.epidermidis, MRSE and B.subtilis were selected for determination. Add different concentrations of tetraphenylethylene derivatives into the five groups of bacterial culture medium, and then add the final concentration of 10 4 CFU / mL of the above-mentioned bacteria, cultured at 37°C for 24 hours and 48 hours, and observed whether the bacteria grow. The lowest concentration of tetraphenylethylene derivatives that can ensure that the bacteria do not grow is the minimum inhibitory concentration of the compound. Smear the bacterial solution with the minimum inhibitory concentration and 2 times, 4 times, and 8 times the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com