Removal method of carboxylic acid in aqueous material containing carboxylic acid impurity
A carboxylic acid removal technology, applied in chemical instruments and methods, water pollutants, water/sewage treatment, etc., can solve the problems of low deacidification efficiency, saturation failure of adsorbents, etc., and achieve good technical effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Catalyst preparation
[0028] 1) Add 0.75 mol MgSO 4 and 0.125 mol Al 2 (SO 4 ) 3 Dissolve in 1 L of water to prepare a uniform mixed salt solution; add dropwise sodium carbonate and sodium hydroxide mixed alkali solution (the concentration of sodium carbonate is 2.5 mol / L, and the concentration of sodium hydroxide is 1.25 mol / L, that is, sodium carbonate and hydrogen The molar ratio of sodium oxide is 2), and the pH value of the control reaction solution is 8.5.
[0029] 2) Aging at room temperature for 6 hours, raising the temperature to 130 °C, keeping it for 24 hours, quenching, filtering, washing with deionized water, and drying at 120 °C overnight to obtain a nano-sized magnesium aluminum hydrotalcite precursor.
[0030] 3) Combine step 2) magnesium aluminum hydrotalcite precursor, 2.5 grams of 30wt% silica sol, 116 grams of 42wt% Mg(Ac) 2 Aqueous solution (i.e. 0.35 mol Mg(Ac) 2 , that is, the ratio of step 1) the number of moles of Mg salt in terms of M...
Embodiment 2
[0039] 1. Catalyst preparation
[0040] According to the catalyst preparation steps in Example 1, a cylindrical (length×diameter: 5 mm×2 mm) magnesium-aluminum composite oxide catalyst was still obtained.
[0041] 2. Catalyst evaluation
[0042] Take 5 grams of the above-mentioned catalyst, and investigate its effect on removing carboxylic acid in the raw material in a fixed-bed continuous microreactor.
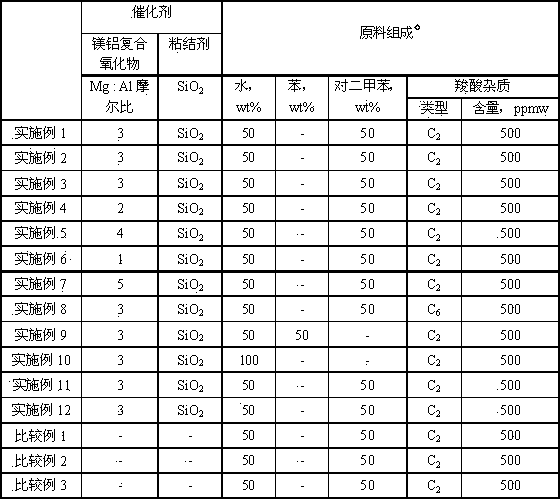
[0043] Raw material composition: water 50wt%, p-xylene 50wt%; carboxylic acid content 500 ppmw, wherein carboxylic acid is acetic acid.
[0044] Specific investigation conditions: reaction pressure, 0.5 MPa; reaction temperature, 400 °C; raw material mass space velocity, 2 h -1 .
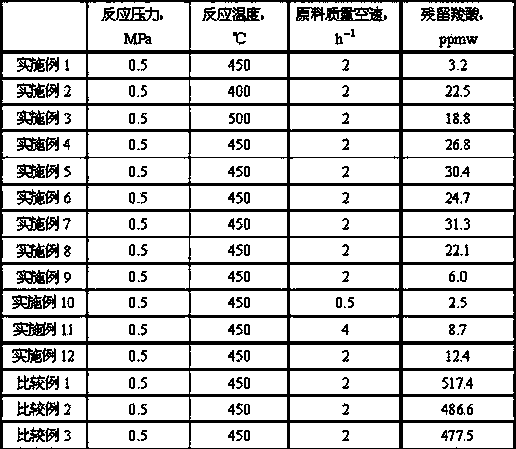
[0045] The amount of residual carboxylic acid in the reaction product was determined by ion chromatography to be 22.5 ppmw.
[0046] For the convenience of comparison, the catalyst composition, raw material composition, reaction pressure, reaction temperature, raw material mass space velocity, ...
Embodiment 3
[0049] 1. Catalyst preparation
[0050] According to the catalyst preparation steps in Example 1, a cylindrical (length×diameter: 5 mm×2 mm) magnesium-aluminum composite oxide catalyst was still obtained.
[0051] 2. Catalyst evaluation
[0052] Take 5 grams of the above-mentioned catalyst, and investigate its effect on removing carboxylic acid in the raw material in a fixed-bed continuous microreactor.
[0053] Raw material composition: water 50wt%, p-xylene 50wt%; carboxylic acid content 500 ppmw, wherein carboxylic acid is acetic acid.
[0054] Specific investigation conditions: reaction pressure, 0.5 MPa; reaction temperature, 500 °C; raw material mass space velocity, 2 h -1 .
[0055] The amount of residual carboxylic acid in the reaction product was determined by ion chromatography to be 18.8 ppmw.
[0056] For the convenience of comparison, the catalyst composition, raw material composition, reaction pressure, reaction temperature, raw material mass space velocity, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com