Gold-iridium bifunctional oxygen electrode catalyst, preparation method and applications thereof
A catalyst and dual-function technology, applied in the field of electrocatalyst, can solve the problems of poor activity, scarce reserves, high price, etc., and achieve the effects of accelerating the industrialization process, benefiting environmental protection, and simple and easy preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
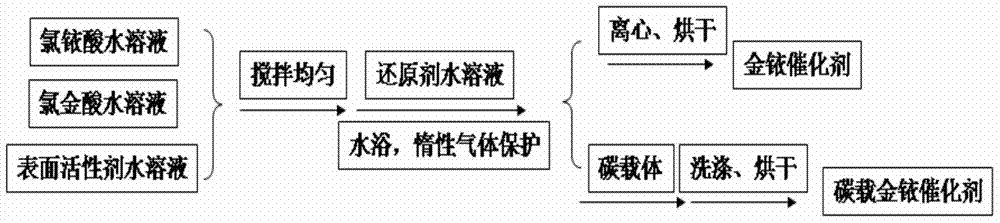
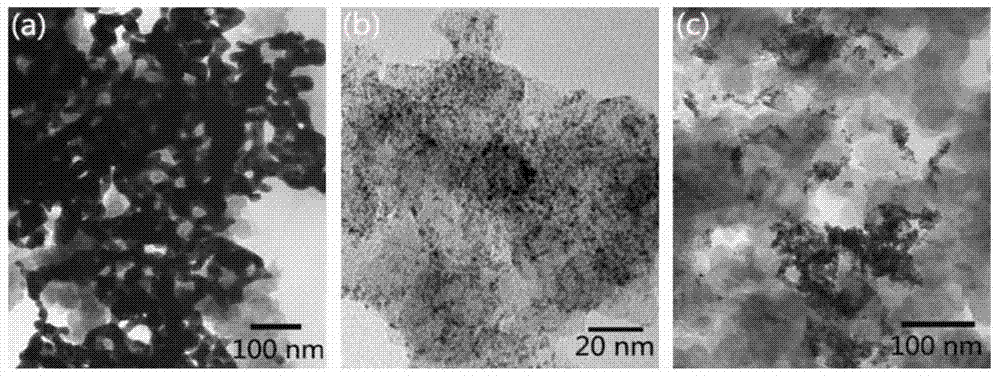
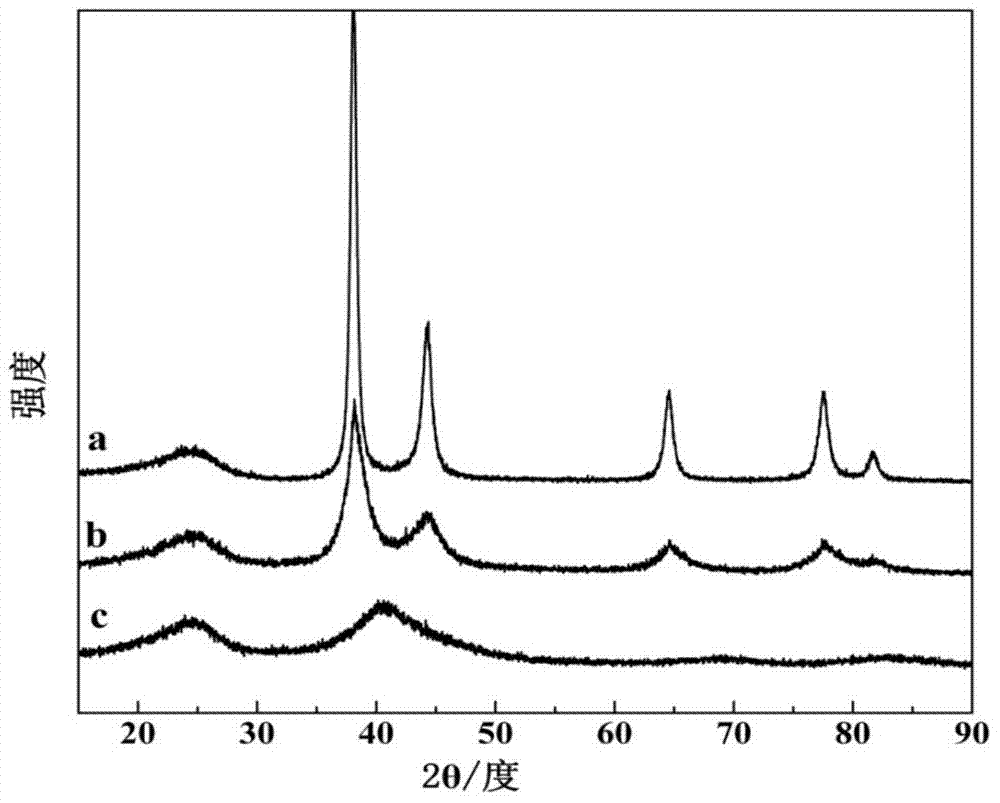
[0036] Add 2.789 mL of 10 mg / mL chloroauric acid aqueous solution and 1.787 mL of 10 mg / mL chloroiridic acid aqueous solution to a three-necked flask containing 100 mL of deionized water and stir evenly, then add 300 mg of hexadecyltrimethyl bromide Ammonium chloride was mixed evenly to obtain a catalyst precursor slurry; the three-necked flask was placed in a 0°C water bath, and nitrogen was used as a protective gas, and 50 mL of sodium borohydride solution that was 1 mg / mL was added to the obtained catalyst precursor slurry to react. After 30 minutes, 80 mg of Vulcan XC-72R was added, and the resulting solution was filtered to obtain a black paste substance; washed with deionized water, and vacuum-dried to obtain a gold-iridium bifunctional oxygen electrode catalyst. The mass content of Au in the catalyst is 13.33%, and the mass content of Ir is 6.67%.
Embodiment 2
[0038] Add 2.091 mL of 10 mg / mL chloroauric acid aqueous solution and 2.680 mL of 10 mg / mL chloroiridic acid aqueous solution to a three-necked flask containing 100 mL of deionized water and stir evenly, then add 300 mg of hexadecyltrimethyl chloride Mix ammonium chloride evenly to obtain a catalyst precursor slurry; place the three-necked flask in a 30°C water bath, and pass argon as a protective gas, and add 25mL of potassium borohydride solution with a concentration of 4.5mg / mL to the obtained catalyst precursor slurry , reacted for 3 hours, and added 80 mg Vulcan XC-72R, and the resulting solution was filtered to obtain a black paste substance; washed with deionized water, and vacuum-dried to obtain a gold-iridium bifunctional oxygen electrode catalyst. The mass content of Au in the catalyst is 10%, and the mass content of Ir is 10%.
Embodiment 3
[0040] Add 1.394mL of 10mg / mL chloroauric acid aqueous solution and 3.573mL of 10mg / mL chloroiridic acid aqueous solution to a three-necked flask filled with 100mL of deionized water and stir evenly, then add 470mg of tetradecyl trimethyl bromide Ammonium chloride was mixed evenly to obtain a catalyst precursor slurry; the three-necked flask was placed in a 70°C water bath, and helium was used as a protective gas, and 25 mL of formaldehyde solution with a concentration of 3.5 mg / mL was added to the obtained catalyst precursor slurry to react. After 12 hours, 80mg of Black Pearls-2000 was added, and the resulting solution was filtered to obtain a black paste substance; washed with deionized water, and vacuum-dried to obtain a gold-iridium bifunctional oxygen electrode catalyst. The mass content of Au in the catalyst is 6.67%, and the mass content of Ir is 13.33%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com