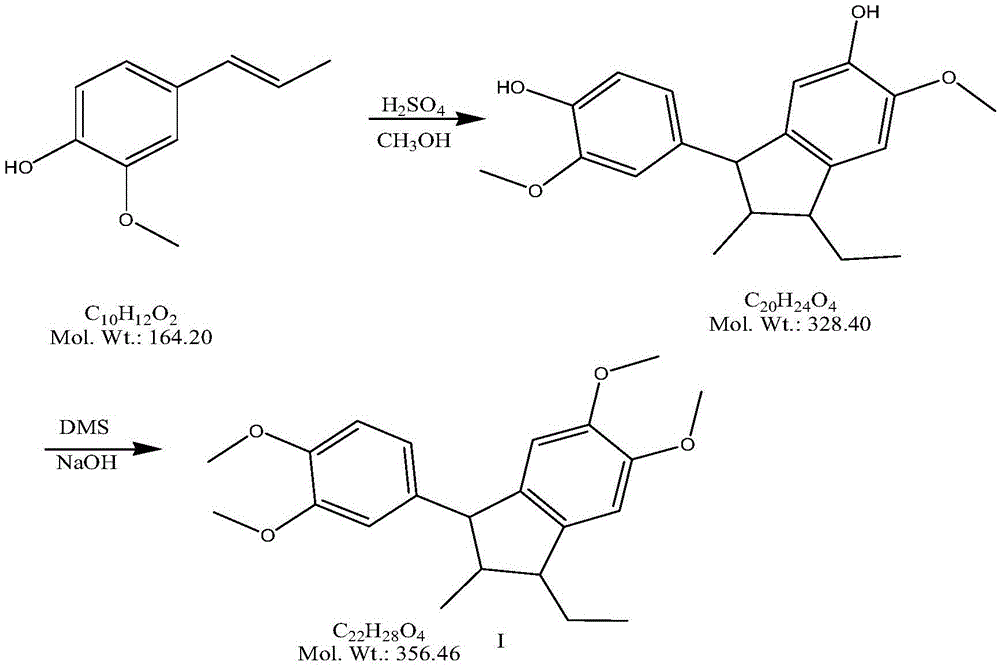
The preparation method of tofisopam intermediate
A technology of tofisopam and intermediates, applied in the field of drug synthesis, can solve the problems of serious pollution and restriction of tofisopam production, and achieve the effect of protecting the environment and reducing the content of heavy metals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
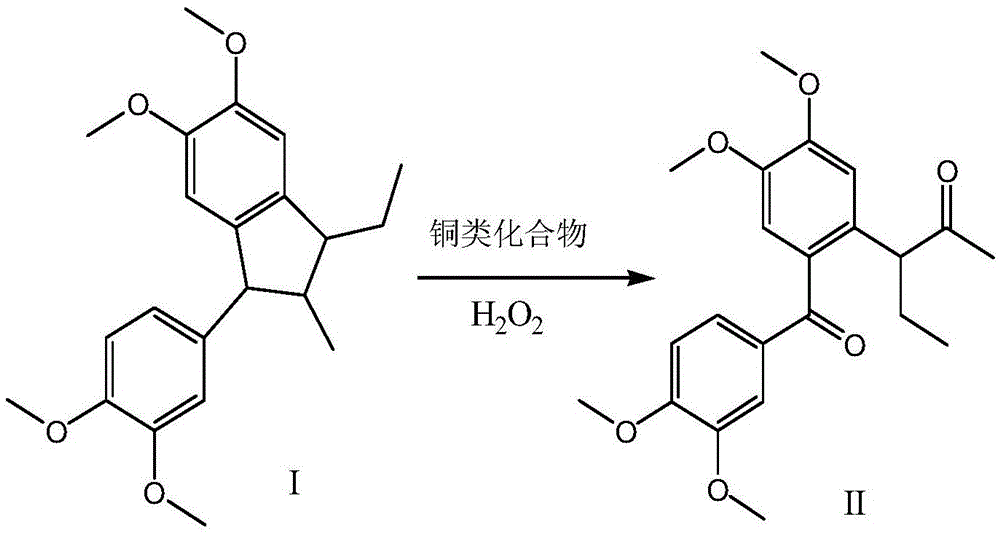
[0031] Add 35.7g (0.10mol) of 1-(3,4-dimethoxy-phenyl)-3-ethyl-5,6-dimethoxy-2-methyl-indane and 357g of water to 500ml In the four-neck flask, add 50.0g (0.5mol) of 98wt% concentrated sulfuric acid, stir; add 0.48g (0.003mol) of copper sulfate, cool down to 3±2°C, add dropwise 17.0g (0.20mol) of 40wt% hydrogen peroxide, and control the temperature At 20±5°C, the dropwise addition is completed, and the temperature is raised to 50±5°C for 1 hour; 200ml of dichloromethane is added for extraction, and the dichloromethane is evaporated to dryness; 150ml of isopropanol is added for recrystallization to obtain 3-[2-(3, 31.7 g of 4-dimethoxybenzoyl)-4,5-dimethoxyphenyl]-pentan-2-one (purity 99.1%), yield 85.6%.
Embodiment 2
[0033] 35.7 g (0.10 mol) of 1-(3,4-dimethoxy-phenyl)-3-ethyl-5,6-dimethoxy-2-methyl-indane and 178.5 g of water were added to In a 500ml four-necked bottle, add 9.7g (0.1mol) of 65wt% concentrated nitric acid, stir; add 0.27g (0.002mol) of copper chloride, cool to 3±2°C, add dropwise 102.0g (0.30mol) of 10wt% hydrogen peroxide, Control the temperature at 25±5°C, after the dropwise addition, raise the temperature to 40±5°C for 5 hours; add 200ml of dichloromethane for extraction, evaporate the methylene chloride to dryness; add 150ml of isopropanol for recrystallization to obtain 3-[2-( 27.8 g of 3,4-dimethoxybenzoyl)-4,5-dimethoxyphenyl]-pentan-2-one (purity 98.9%), yield 75.1%.
Embodiment 3
[0035] Add 35.7g (0.10mol) 1-(3,4-dimethoxy-phenyl)-3-ethyl-5,6-dimethoxy-2-methyl-indane and 215g water to 500ml In the four-neck flask, add 19.8g (0.2mol) of 36.8wt% concentrated hydrochloric acid, 12g (0.2mol) of glacial acetic acid, and stir; add 0.4g (0.005mol) of copper oxide, cool down to 3±2°C, and dropwise add 30wt% hydrogen peroxide 56.7g (0.50mol), control the temperature at 15±5°C, after the dropwise addition is complete, raise the temperature to 25±5°C for 10 hours; add 200ml of dichloromethane for extraction, evaporate the dichloromethane to dryness; add 150ml of ethanol for recrystallization, and obtain 3 -[2-(3,4-Dimethoxybenzoyl)-4,5-dimethoxyphenyl]-pentan-2-one 32.7g (purity 99.4%), yield 88.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com