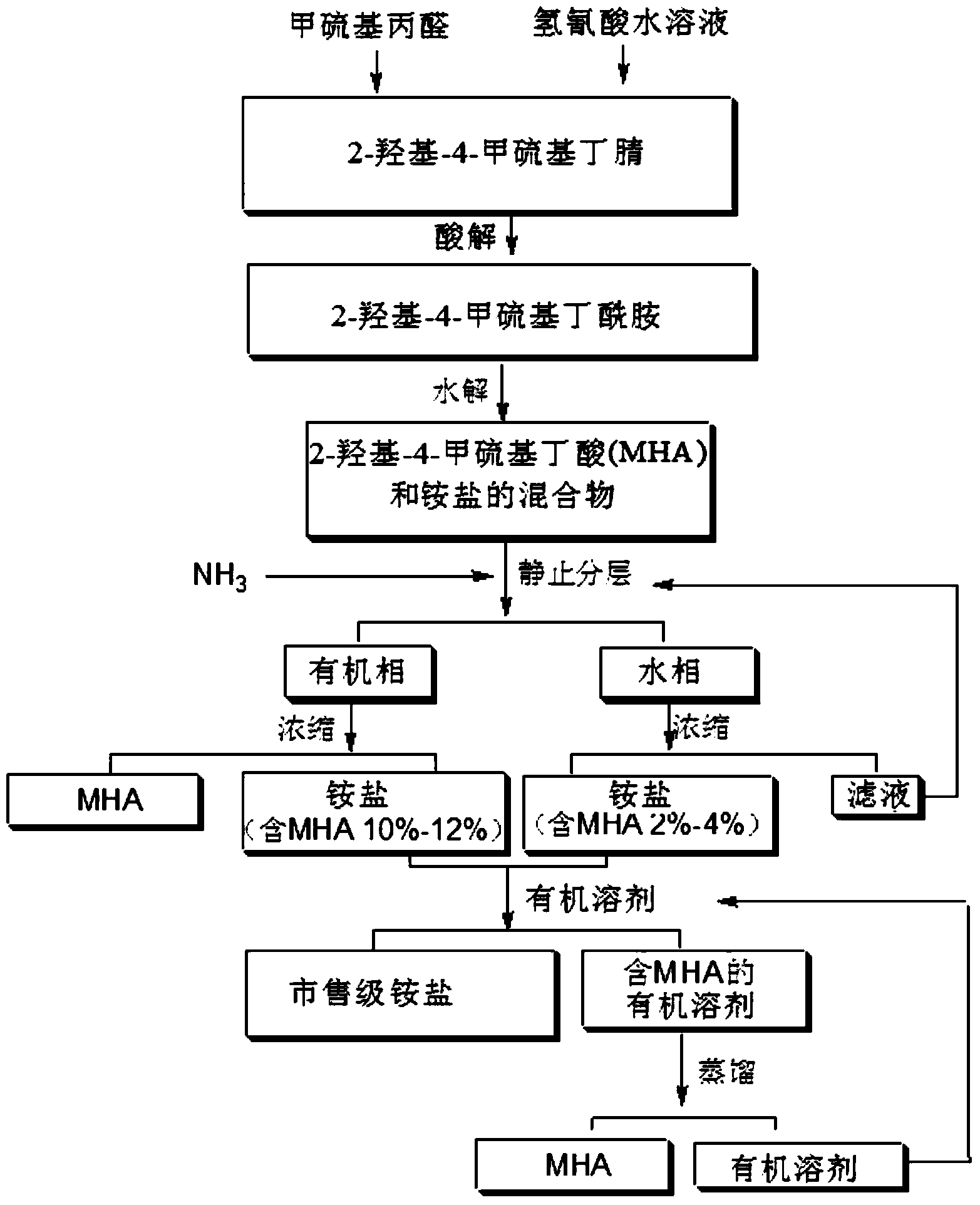
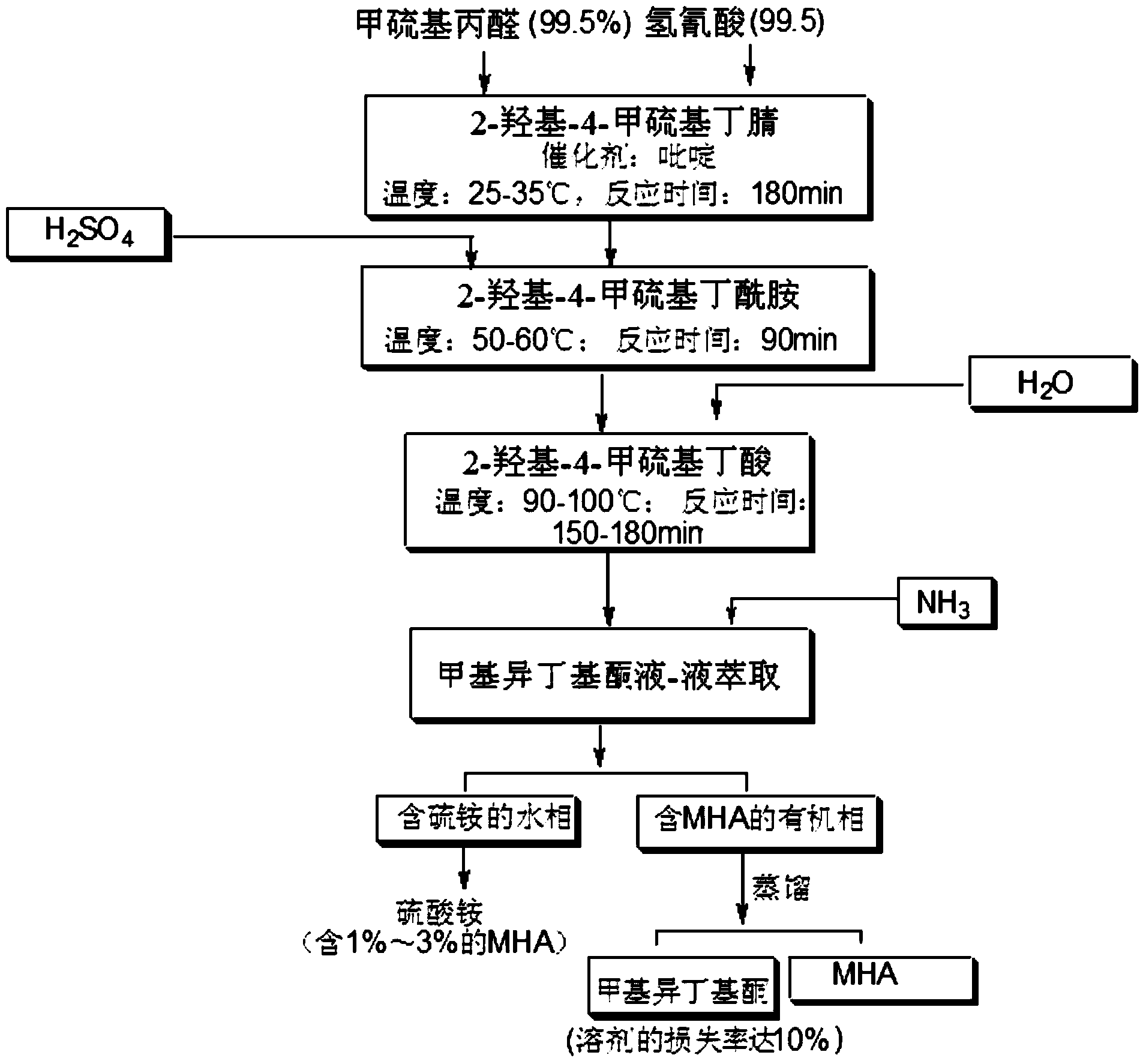
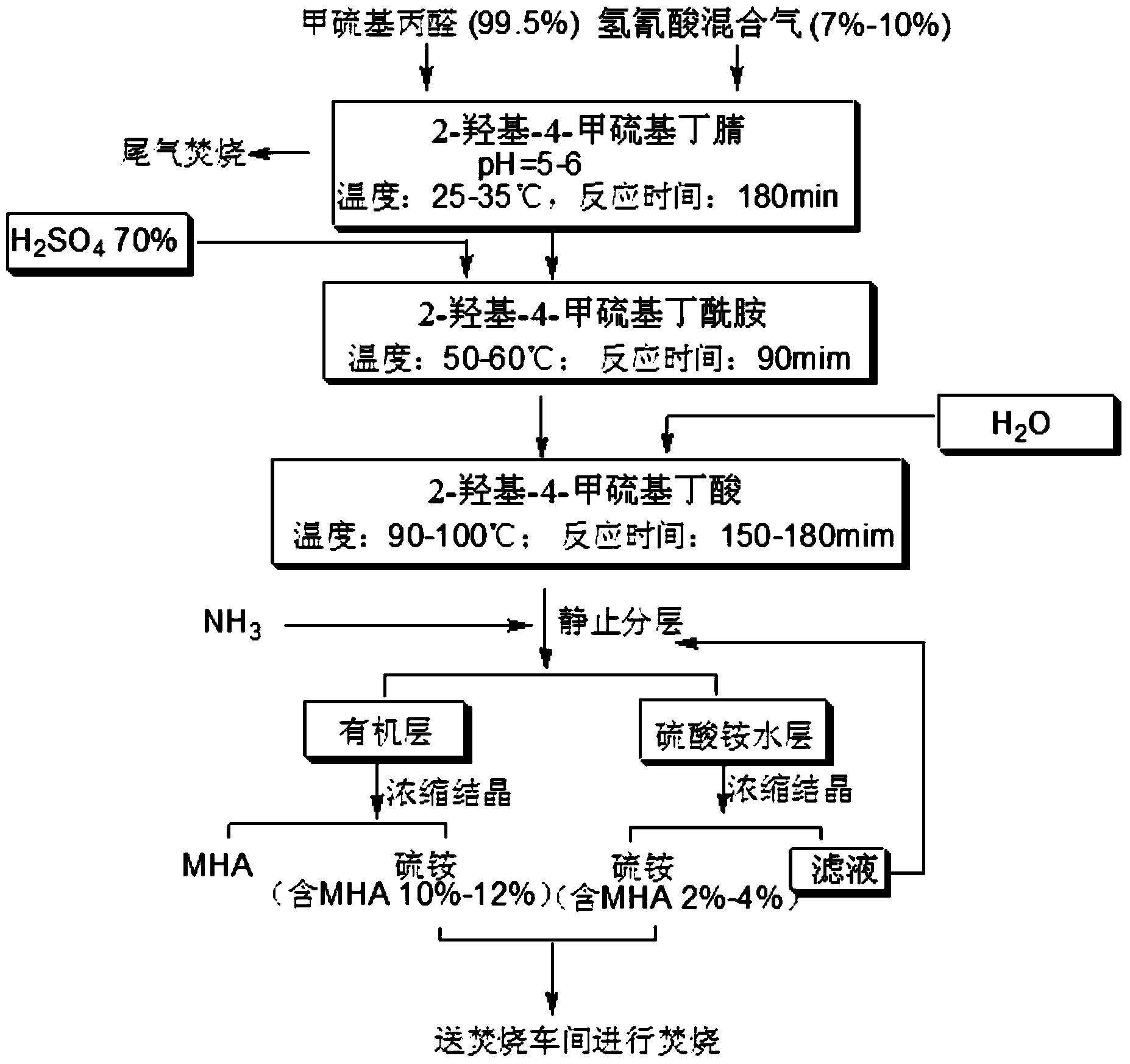
Preparation method of MHA (2-hydroxy-4-(methylthio) butyric acid)
A technology of methylthiobutyric acid and methylthiobutyronitrile is applied in the preparation of sulfide, the preparation of organic compounds, ammonium sulfate and other directions, and can solve the problems of large consumption of raw materials, serious environmental pollution, increased energy consumption and the like, Achieve the effect of reducing usage and loss, reducing production costs and reducing losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] In a coolable reactor with a pH electrode and a thermometer, add 420.2 g of methionaldehyde (4 mol) with a mass fraction of 99%, and adjust the pH value to 5.0 to 5.5 with citric acid and sodium citrate buffer , stirred vigorously at 30°C, cooled, slowly added dropwise 189g, and the mass fraction was 60% hydrocyanic acid aqueous solution (4.2mol), the control reaction temperature was not more than 40°C, and when adding hydrocyanic acid aqueous solution, continuously added citric acid and Sodium citrate buffer to maintain the pH around 5.5. After hydrocyanic acid was added, the cooling bath was removed, and then stirred at 30°C for 1 h to obtain 611.2 g of 2-hydroxy-4-methylthiobutyronitrile with a mass fraction of 85.83%, to which 98% mass fraction was added Sulfuric acid makes pH=3. Based on the added methional, the reaction yield is 99.96%.
Embodiment 2
[0053] In a coolable reactor with a pH electrode and a thermometer, add 420.2 g of methionaldehyde (4 mol) with a mass fraction of 99%, and adjust the pH value to 5.0 to 6.0 with citric acid and sodium citrate buffer . Stir vigorously at 30°C, cool, slowly add 283.5g dropwise, with a mass fraction of 40% hydrocyanic acid aqueous solution (4.2mol), control the reaction temperature not to exceed 40°C, and continuously add citric acid and Sodium citrate buffer to maintain the pH around 5.5. After the hydrocyanic acid was added, the cooling bath was removed, and then stirred at 30°C for 1 h to obtain 703.7 g of 2-hydroxy-4-methylthiobutyronitrile with a mass fraction of 74.2%. Phosphoric acid with a mass fraction of 85% was added therein to make it pH=3. Based on the added methional, the reaction yield is 99.95%.
Embodiment 3
[0055] In a coolable reactor with a pH electrode and a thermometer, add 433.33 g of methionaldehyde (4 mol) with a mass fraction of 96%, and adjust the pH value to 5.0 to 6.0 with citric acid and sodium citrate buffer . Stir vigorously at 30°C, cool, slowly add 378g of hydrocyanic acid aqueous solution (4.2mol) with a mass fraction of 30%, and control the reaction temperature not to exceed 40°C. When adding hydrocyanic acid aqueous solution, continuously add citric acid and lemon sodium bicarbonate buffer to maintain the pH around 5.5. After the hydrocyanic acid was added, the cooling bath was removed, and then stirred at 30°C for 1 h to obtain 811.3 g of 2-hydroxy-4-methylthiobutyronitrile with a mass fraction of 64.2%. Phosphoric acid with a mass fraction of 85% was added therein to make it pH=3. Based on the added methional, the reaction yield is 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com