Preparation method of olmesartan medoxomil
A technology of olmesartan medoxomil and benzyl olmesartan medoxomil is applied in the fields of pharmaceutical preparation and medicinal chemistry, and can solve problems such as unfavorable energy saving, emission reduction and environmental protection, complicated operation, increased production cost and sewage treatment cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
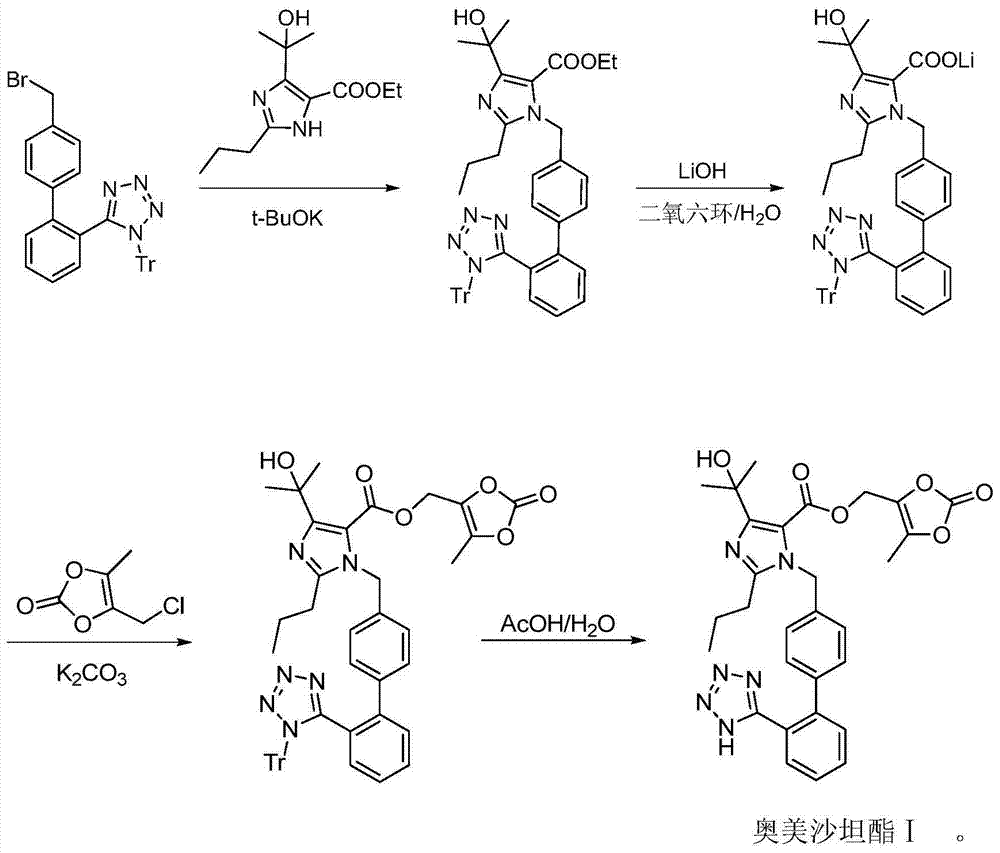
[0033] 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{4-[2-(trityltetrazol-5-yl)phenyl]phenyl}methylimidazole- Preparation of ethyl 5-carboxylate
[0034]
[0035] Add 1-trityl-5-(4'-bromomethylbiphenyl-2-)-1H-tetrazole (500 g, 0.9 mol), 4-(1-hydroxy-1-methyl) to the autoclave ethyl)-2-propylimidazole-5-carboxylic acid ethyl ester (180g, 0.75mol), potassium carbonate (390g), and then acetonitrile (7.5L) was added, and the reaction was heated under reflux for 3 to 5 hours. After monitoring the completion of the reaction by TLC, it was cooled to room temperature (25° C.), filtered, and the filtrate was concentrated to dryness at 40° C. under reduced pressure (-0.09 MPa). Methyl tert-butyl ether (1L) was added, stirred at room temperature (25°C) for crystallization for 2 hours, filtered and dried at 60°C under reduced pressure (-0.09MPa) to obtain a solid compound 4-(1-hydroxy-1-methylethyl) )-2-propyl-1-{4-[2-(trityltetrazol-5-yl)phenyl]phenyl}methylimidazole-5-carboxylate (440 g)...
Embodiment 2
[0037] 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{4-[2-(trityltetrazol-5-yl)phenyl]phenyl}methylimidazole- Preparation of lithium 5‐carboxylate and trityl olmesartan medoxomil VII
[0038]
[0039] Add 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{4-[2-(trityltetrazol-5-yl)phenyl]phenyl to the kettle } Methylimidazole-5-carboxylate ethyl ester (358g, 0.5mol), lithium hydroxide (32g, 0.75mol), dioxane (8L) and water (4L), warm the reaction to 55°C, stir the reaction After 5 to 7 hours, after monitoring the completion of the reaction by TLC, the reaction system was cooled to 25° C., 2 L of ethyl acetate and 1 L of saturated brine were added to the reaction solution, and the layers were separated. The aqueous phase was extracted three times with ethyl acetate, each 2L, the organic phases were combined, and the organic phases were washed three times with saturated brine, then the organic phase was dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated at 40°...
Embodiment 3
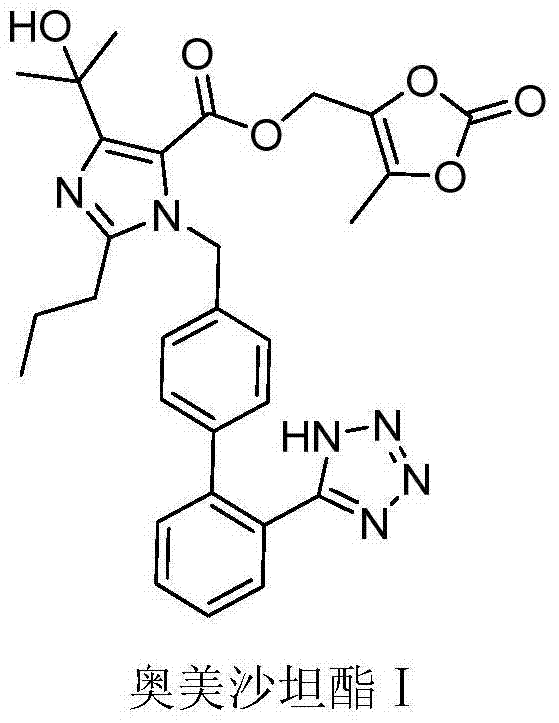
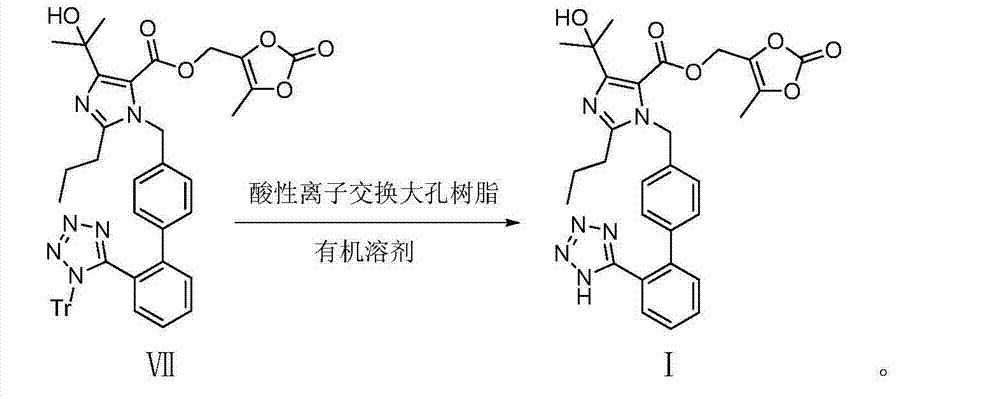
[0041] 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{4-[2-(tetrazol-5-yl)phenyl]phenyl}methylimidazole-5-carboxylic acid Preparation of (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl ester (olmesartan medoxomil I)
[0042]
[0043] Trityl olmesartan medoxomil VII (800 mg, 1 mmol) in the reaction flask was dissolved in 4 mL of dichloromethane and 4 mL of methanol mixed solvent, 400 mg of acidic ion-exchange macroporous resin Amberlyst-15 was added, the reaction was stirred at room temperature, and the reaction was monitored by TLC After completion, filter, wash the resin with methanol, monitor the washings by TLC until no organics remain, combine the filtrates, and concentrate the filtrates to dryness at 40°C under reduced pressure (-0.09MPa) to obtain an oily substance, add dichloromethane (3mL) and heat to 40°C ℃ dissolved, filtered, the filtrate was concentrated to dryness at 40 ℃ under reduced pressure (-0.09MPa), ethyl acetate (3 mL) was added and heated to dissolve, cooled to 4 ℃ fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com