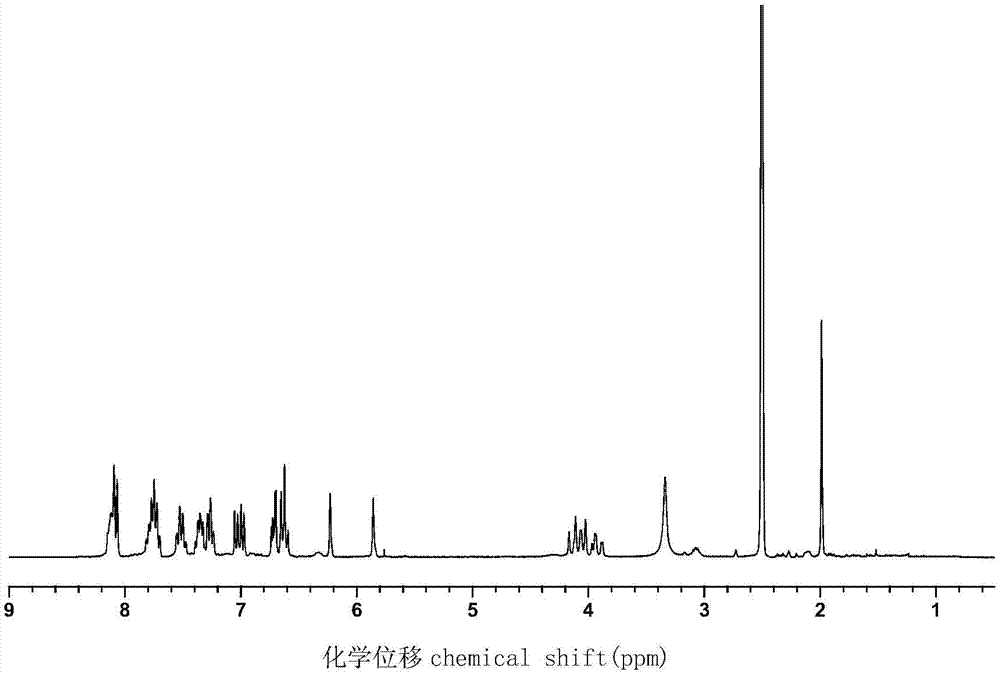
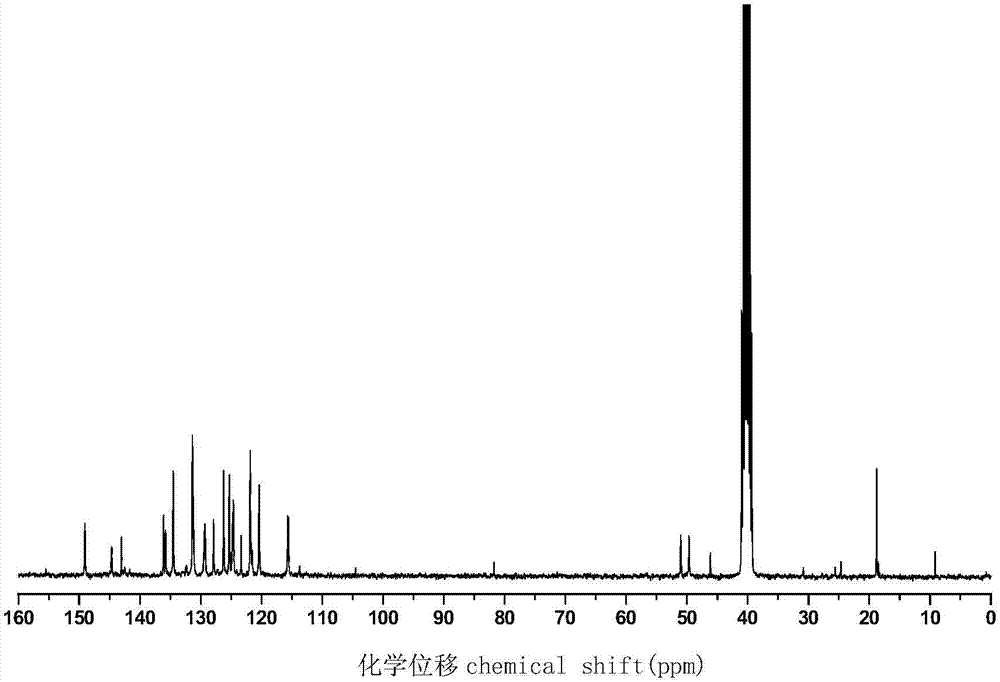
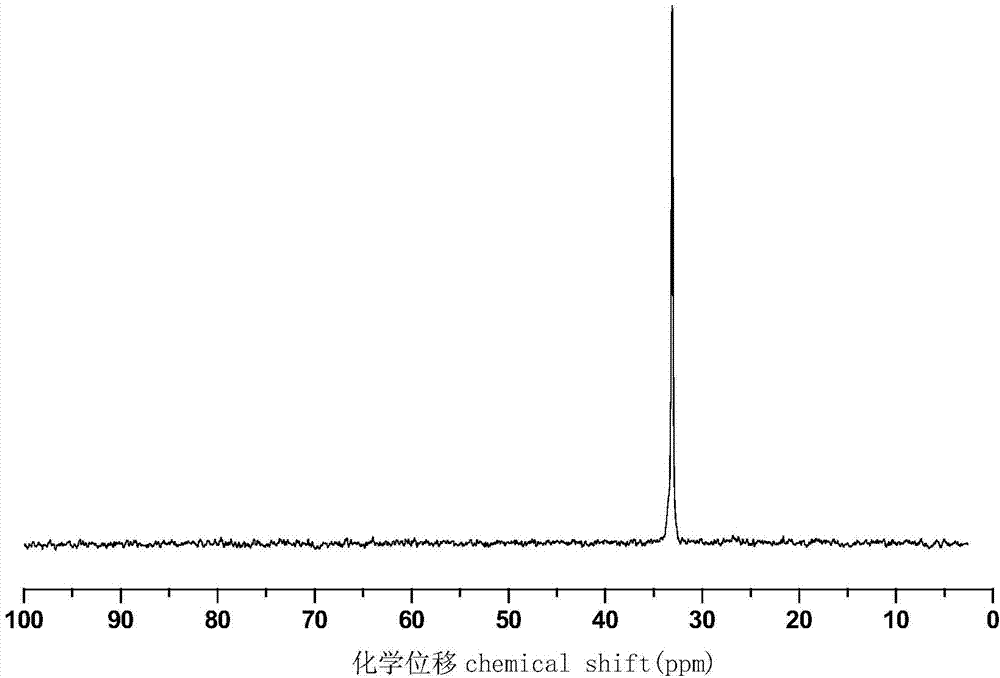
Phosphorus-nitrogen type flame retardant containing active double bond as well as preparation method and application thereof
A phosphorus-nitrogen-based flame retardant and active technology are applied in the field of phosphorus-nitrogen-based flame retardants and their preparation, which can solve the problems of flame retardant loss and product performance deterioration, and achieve good flame retardant effect, low cost, and easy industrialization. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 43.2 g (0.2 mol) of DOPO, 10.9 g (0.1 mol) of p-aminophenol, 6.0 g (0.1 mol) of paraformaldehyde and 200 mL of ethanol into a 500 mL three-necked round bottom flask, slowly raise the temperature to 50 °C and stir And reflux reaction for 5h, and then the crude product was obtained after the solvent was removed by rotary evaporation, and the phenolic intermediate compound was obtained after repeated washing with methanol three times and drying, with a yield of about 95%.
[0028] Add 33.2g (0.05mol) of the previously synthesized phenolic intermediate compound, 10.1g (0.10mol) of triethylamine and 100mL of dichloromethane to a 250mL single-necked round-bottomed flask in sequence, place it at 5°C, and slowly 10.5 g (0.10 mol) of methacryloyl chloride was added dropwise, and stirring was continued for 8 hours after the dropwise addition was completed. After the reaction was completed, triethylamine hydrochloride was removed by filtration, and washed several times with 3%...
Embodiment 2
[0030] Add 43.2 g (0.2 mol) of DOPO, 10.9 g (0.1 mol) of p-aminophenol, 6.0 g (0.1 mol) of paraformaldehyde and 200 mL of tetrahydrofuran into a 500 mL three-necked round-bottomed flask, slowly heat up to 65 °C and stir And reflux reaction for 4h, and then the crude product was obtained after the solvent was removed by rotary evaporation, and the phenolic intermediate compound was obtained after repeated washing with methanol three times and drying, with a yield of about 88%.
[0031]In a 250mL single-necked round bottom flask, add 33.2g (0.05mol) of previously synthesized phenolic intermediate compound, 10.1g (0.10mol) of triethylamine and 100mL of dichloromethane in sequence, place it at 20°C, and slowly 7.9 g (0.10 mol) of methacryloyl chloride was added dropwise, and stirring was continued for 6 h after the dropwise addition was completed. After the reaction was completed, pyridine hydrochloride was removed by filtration, and washed several times with 3% NaOH solution, dis...
Embodiment 3
[0033] Add 43.2 g (0.2 mol) of DOPO, 10.9 g (0.1 mol) of p-aminophenol, 6.0 g (0.1 mol) of paraformaldehyde and 200 mL of acetonitrile into a 500 mL three-necked round-bottomed flask, slowly raise the temperature to 80 °C and stir And reflux reaction for 3h, then the crude product was obtained after the solvent was removed by rotary evaporation, and the phenolic intermediate compound was obtained after repeated washing with methanol three times and drying, with a yield of about 92%.
[0034] Add 33.2g (0.05mol) of the previously synthesized phenolic intermediate compound, 10.1g (0.10mol) of triethylamine and 100mL of toluene to a 250mL single-necked round-bottomed flask in sequence, place it at 40°C, and slowly drop 10.5g (0.10mol) of methacryloyl chloride, after the dropwise addition, continue to stir and react for 4h. The back was washed several times with 3% NaOH solution, distilled water, and saturated NaCl aqueous solution successively. Anhydrous MgSO for organic layer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com