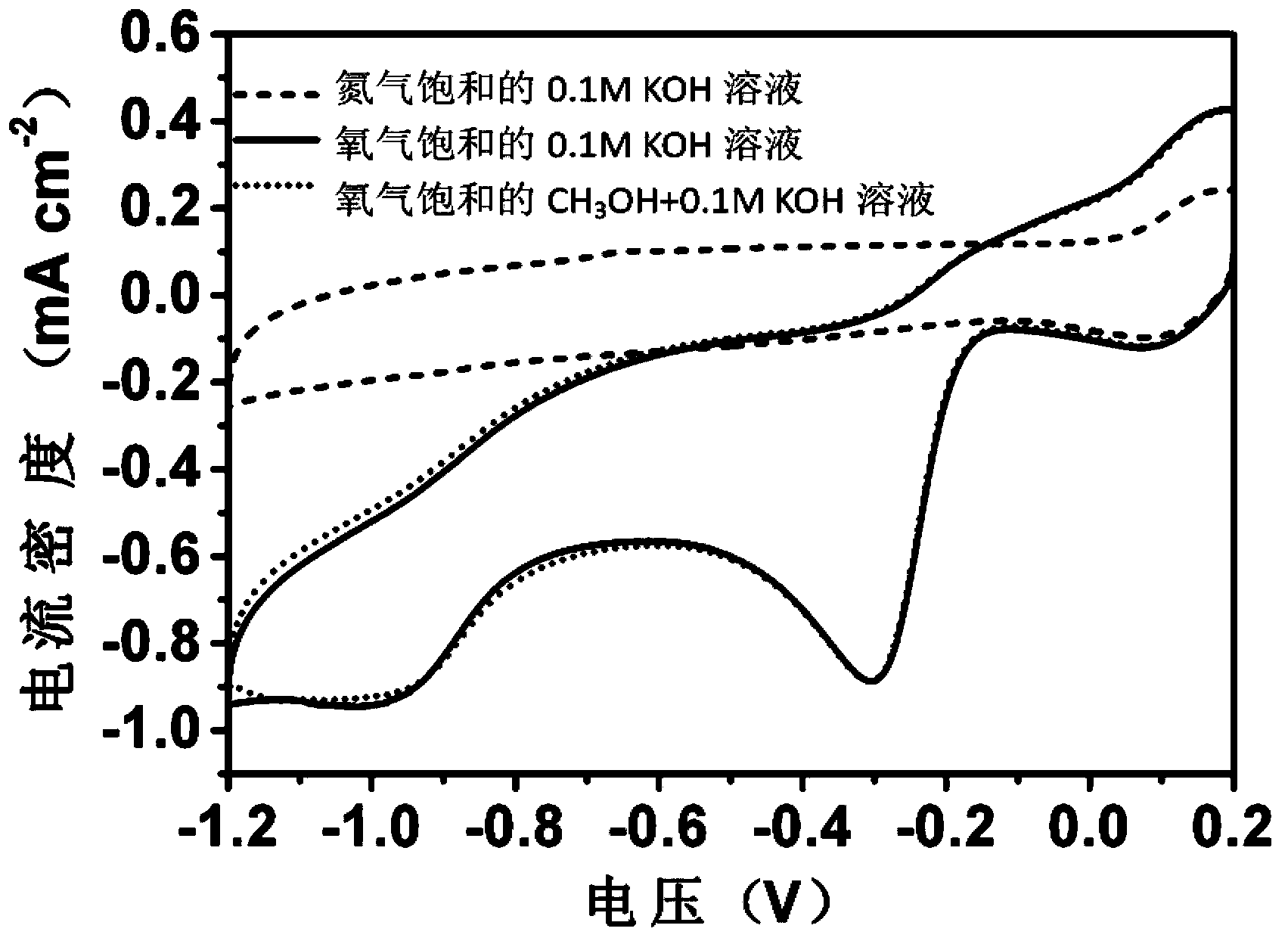
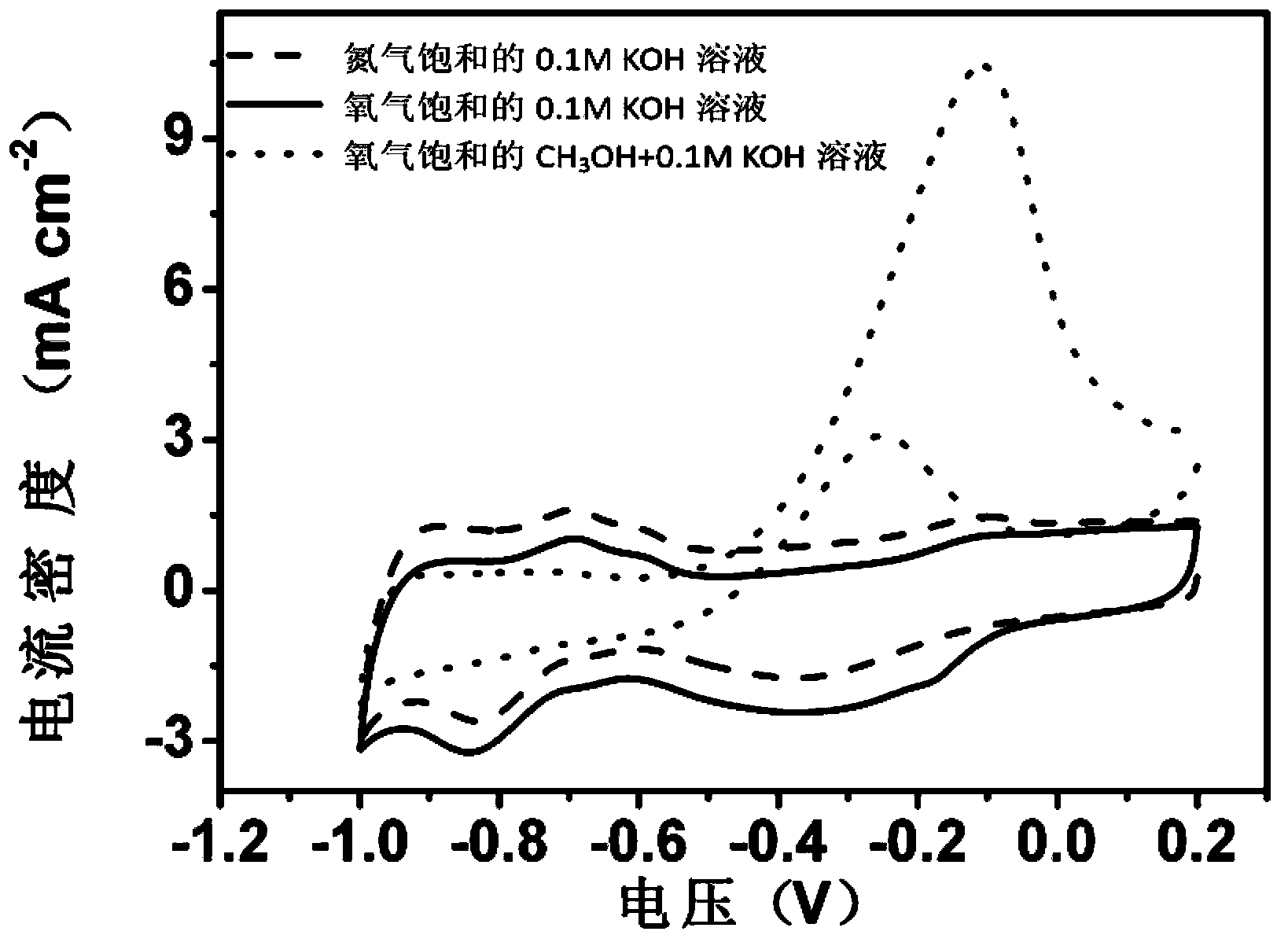
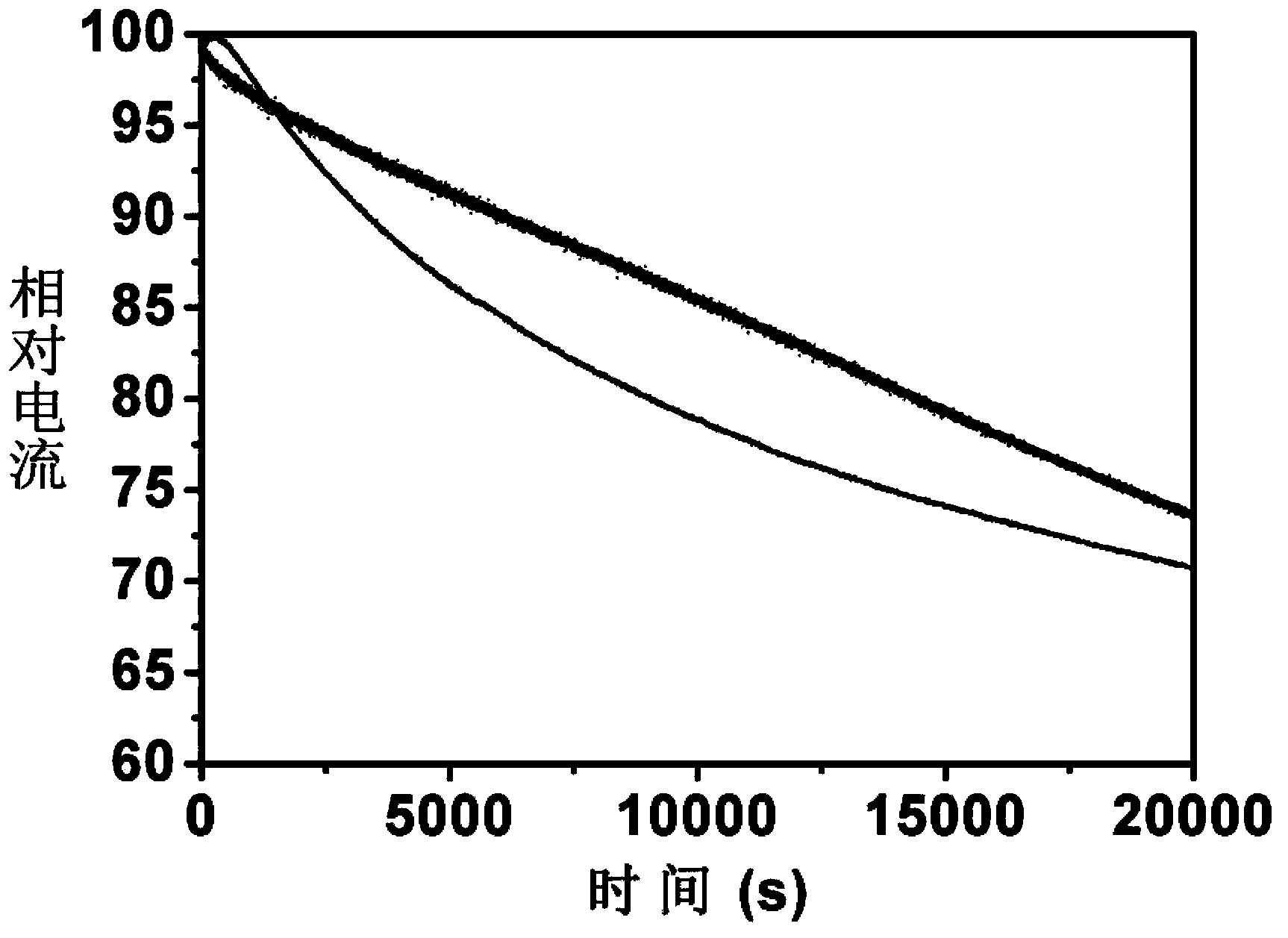
Preparation method of metal-free oxygen reduction catalyst
A catalyst and metal-free technology, applied in structural parts, electrical components, battery electrodes, etc., can solve the problems of high-temperature equipment, high energy consumption, complex process, etc., and achieve the effects of low energy consumption, good stability, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1, comprises the steps:
[0020] In step (1), the glassy carbon substrate is polished to a mirror surface on a polishing cloth with 1.0 μm and 0.3 μm alumina powder in turn, and then used as a working electrode, platinum wire as a counter electrode, Ag / Ag + As a reference electrode, the electrochemical oxidation reaction is carried out in the electrolyte, and the potential of the electrochemical oxidation reaction is 0V to 1.2V, and a glassy carbon substrate with a negative charge on the surface is obtained;
[0021] The electrolyte is a mixed solution of 4-aminobenzoic acid, lithium perchlorate and absolute ethanol, and the molar concentrations of 4-aminobenzoic acid and lithium perchlorate in the mixed solution are 1mM and 0.1M respectively;
[0022] In step (2), immerse the negatively charged glassy carbon substrate obtained in step (1) on its surface in a 0.2 wt% aqueous solution of diethylene glycol diacrylate (PDDA) by mass, and take it out after 5 minu...
Embodiment 2
[0026] Embodiment 2, comprises the steps:
[0027] In step (1), the glassy carbon substrate is polished to a mirror surface on a polishing cloth with 1.0 μm and 0.3 μm alumina powder in turn, and then used as a working electrode, platinum wire as a counter electrode, Ag / Ag + As a reference electrode, the electrochemical oxidation reaction is carried out in the electrolyte, and the potential of the electrochemical oxidation reaction is 0V to 1.2V, and a glassy carbon substrate with a negative charge on the surface is obtained;
[0028] The electrolyte is a mixed solution of 4-aminobenzoic acid, lithium perchlorate and absolute ethanol, and the molar concentrations of 4-aminobenzoic acid and lithium perchlorate in the mixed solution are 3mM and 0.3M respectively;
[0029]In step (2), immerse the negatively charged glassy carbon substrate obtained in step (1) on its surface in an aqueous solution of 0.5 wt% diethylene glycol diacrylate (PDDA) by mass percentage, take it out after...
Embodiment 3
[0037] Embodiment 3, comprises the steps:
[0038] In step (1), the glassy carbon substrate is polished to a mirror surface on a polishing cloth with 1.0 μm and 0.3 μm alumina powder in turn, and then used as a working electrode, platinum wire as a counter electrode, Ag / Ag + As a reference electrode, the electrochemical oxidation reaction is carried out in the electrolyte, and the potential of the electrochemical oxidation reaction is 0V to 1.2V, and a glassy carbon substrate with a negative charge on the surface is obtained;
[0039] The electrolyte is a mixed solution of 4-aminobenzoic acid, lithium perchlorate and absolute ethanol, and the molar concentrations of 4-aminobenzoic acid and lithium perchlorate in the mixed solution are 7mM and 0.4M respectively;
[0040] In step (2), the negatively charged glassy carbon substrate obtained in step (1) is immersed in a 0.8 wt% aqueous solution of diethylene glycol diacrylate (PDDA) by mass, and taken out after 30 minutes. Rinse ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com